
Adsorption and absorption are two distinct processes that involve the interaction of substances, particularly in chemistry, physics, and industrial applications. While adsorption refers to the adhesion of molecules onto a surface, absorption involves the uptake of one substance into the bulk of another. This article explores the fundamental differences between adsorption vs. absorption, their mechanisms, examples, and real-world applications.
What is Adsorption?
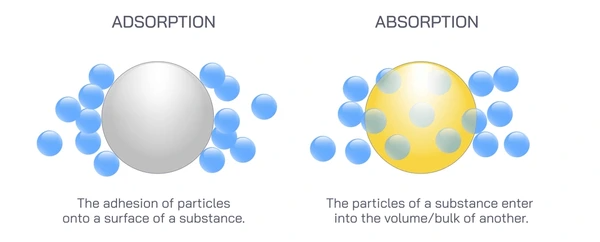
Adsorption is the process in which molecules, atoms, or ions adhere to the surface of a solid or liquid without penetrating its internal structure. This phenomenon is widely used in industrial filtration, catalysis, and material science.
How Adsorption Works
- Occurs when gas or liquid molecules accumulate on a surface due to van der Waals forces or chemical bonding.
- Can be classified into two types: physisorption (weak physical attraction) and chemisorption (strong chemical bonding).
- The adsorbed molecules form a thin layer on the solid’s surface without becoming part of its bulk.
Examples of Adsorption
- Activated carbon in air and water purifiers traps toxins and pollutants on its surface.
- Silica gel absorbs moisture by attracting water molecules to its surface.
- Catalysts in chemical reactions rely on adsorption to facilitate reactions.
What is Absorption?
Absorption is the process in which one substance is taken into the volume of another, forming a homogeneous mixture. Unlike adsorption, absorption involves a complete penetration into the absorbing material.
How Absorption Works
- The molecules of the absorbed substance distribute throughout the bulk of the absorbent.
- The process continues until equilibrium is reached between the two substances.
- It is commonly observed in liquids and porous solids.
Examples of Absorption
- A sponge soaking up water distributes the liquid throughout its structure.
- Oxygen dissolving into water in a pond is an example of gas absorption.
- Human skin absorbing lotions and creams involves the penetration of substances into the dermal layers.
Comparison: Adsorption vs. Absorption
Confused about the difference between adsorption and absorption? Eureka Technical Q&A provides expert insights into their distinct mechanisms, applications, and real-world examples, helping you understand which process is best suited for your needs.
| Feature | Adsorption | Absorption |
|---|---|---|
| Definition | Surface-based adhesion of molecules | Bulk penetration of a substance into another |
| Process Type | Occurs at the surface of a material | Occurs throughout the entire volume |
| Energy Requirement | Generally lower energy process | Requires more energy for diffusion and mixing |
| Example in Daily Life | Charcoal filters trapping impurities | Water soaking into a sponge |
| Industrial Application | Catalysis, air purification, gas storage | Liquid and gas separation, refrigeration |
- Surface vs. Volume: Adsorption occurs on the surface of the adsorbent, while absorption involves the entire volume of the absorbing material.
- Energy and Forces: Adsorption can be driven by weak van der Waals forces (physisorption) or strong chemical bonds (chemisorption), whereas absorption is typically driven by physical properties like solubility.
- Process Type: Adsorption is generally a reversible, surface-based process, whereas absorption can be a bulk process that may or may not be reversible depending on the system.
Which Process is More Important?
Both adsorption and absorption are crucial in different fields. Adsorption plays a significant role in filtration, catalysis, and gas storage, while absorption is essential in separation processes, refrigeration, and biological systems.
Choose adsorption if:
- The goal is to trap contaminants or chemical species on a solid surface.
- Applications involve catalysis, pollution control, or gas storage.
Choose absorption if:
- The process requires the complete uptake of a substance into another medium.
- It involves liquids or porous solids, such as in cooling systems and chemical separations.
FAQs
Is adsorption reversible?
Yes, adsorption can be reversible, particularly in physisorption, where weak intermolecular forces hold molecules on a surface. Chemisorption, involving chemical bonds, is often irreversible.
How does temperature affect adsorption and absorption?
Adsorption generally decreases with higher temperatures as increased kinetic energy disrupts surface bonding. Absorption can increase with temperature due to enhanced molecular movement and diffusion.
What is an adsorption isotherm?
An adsorption isotherm describes how the amount of adsorbate on a surface changes with pressure or concentration at a constant temperature, commonly modeled by Langmuir and Freundlich isotherms.
What industries rely on adsorption and absorption?
Adsorption is widely used in pollution control, catalysis, and pharmaceuticals, while absorption is essential in chemical processing, refrigeration, and gas-liquid separation.
How do activated carbon filters use adsorption?
Activated carbon filters have a highly porous surface that adsorbs impurities like chemicals and toxins, effectively removing contaminants from air or water.
Conclusion
Adsorption and absorption are distinct processes with essential applications in science and industry. Adsorption occurs at surfaces and is critical for catalysis and filtration, while absorption involves the full penetration of a substance into another, essential for cooling, separation, and biological functions. Understanding their differences helps in selecting the right process for specific industrial and everyday applications.
To get detailed scientific explanations of adsorption vs. absorption, try Patsnap Eureka.


