
Aldehyde vs. ketone are two fundamental classes of organic compounds that contain the carbonyl group (C=O). Despite their shared functional group, they differ in structure, reactivity, and applications. These compounds are essential in synthetic chemistry, biology, and industry, and understanding their differences is key to mastering organic chemistry. This article explains how aldehydes and ketones differ, how to identify them using lab tests, and where they’re used in real-world applications.
Aldehyde vs. Ketone—what’s the difference? Eureka Technical Q&A explains their distinct structures and reactivity, helping you easily identify and understand their roles in organic chemistry.
What Are Aldehydes and Ketones?
Both aldehydes and ketones contain a carbonyl group—a carbon atom double-bonded to an oxygen atom. The difference lies in what is attached to that carbonyl carbon.
Aldehyde Functional Group
In aldehydes, the carbonyl carbon is bonded to at least one hydrogen atom and one alkyl or aryl group.
General structure: R–CHO
Example: Formaldehyde (HCHO), Acetaldehyde (CH₃CHO)

Ketone Functional Group
In ketones, the carbonyl carbon is bonded to two carbon-based groups (alkyl or aryl).
General structure: R–CO–R’
Example: Acetone (CH₃COCH₃), Butanone (CH₃COCH₂CH₃)
Structural Differences
- Aldehydes: In aldehydes, the carbonyl group is attached to a carbon atom at the end of a carbon chain. This means that an aldehyde has the formula R-CHO, where R represents a hydrogen atom or a hydrocarbon group, and the carbonyl group is located at the terminal position.
- Ketones: In ketones, the carbonyl group is located between two carbon atoms of the carbon chain. The general formula for a ketone is R-CO-R’, where R and R’ can be hydrogen or hydrocarbon groups. This structural difference gives ketones a more internal placement of the carbonyl group compared to aldehydes.
| Feature | Aldehyde | Ketone |
|---|---|---|
| Location of Carbonyl | Terminal (end of carbon chain) | Internal (within the carbon chain) |
| Substitution | One H, one R group | Two R groups |
| General Formula | R–CHO | R–CO–R’ |
Because aldehydes have a hydrogen attached to the carbonyl group, they are generally more reactive than ketones.
Physical Properties
- Aldehydes: Due to the presence of an alpha hydrogen, aldehydes can undergo keto-enol tautomerism and are more reactive in nucleophilic addition reactions. Aldehydes can also be easily oxidized to carboxylic acids.
- Ketones: Ketones are generally less reactive than aldehydes due to the absence of alpha hydrogens. However, they can still participate in nucleophilic addition reactions and can undergo condensation reactions to form larger molecules
| Property | Aldehyde | Ketone |
|---|---|---|
| Boiling Point | Lower than ketones of similar mass | Higher than aldehydes due to greater dipole moment |
| Solubility in Water | Good for small aldehydes | Good for small ketones |
| Odor | Often pungent or irritating | Mild or pleasant (acetone-like) |
Chemical Tests: How to Distinguish Aldehydes from Ketones
Several classic lab tests can help differentiate aldehydes from ketones based on their reactivity.
1. Tollens’ Test
Aldehydes react with Tollens’ reagent to form a silver mirror on the test tube.
Ketones do not react.
2. Fehling’s Test
Aldehydes reduce Fehling’s solution, forming a red precipitate of copper(I) oxide.
Ketones typically do not react.
3. Schiff’s Test
Aldehydes restore the pink color of Schiff’s reagent.
Ketones usually do not affect the reagent.
4. Iodoform Test
Some methyl ketones (like acetone) give a yellow precipitate with iodine and base.
Aldehydes do not respond unless they are easily oxidized to such ketones.
Reactivity and Reactions
Aldehydes
- More reactive due to the electron-withdrawing hydrogen
- Easily oxidized to carboxylic acids
- Undergo nucleophilic addition reactions
Ketones
- Less reactive than aldehydes
- Resistant to mild oxidation
- Also undergo nucleophilic addition, especially with Grignard reagents and hydrides
Common Uses and Applications
Aldehydes
- Formaldehyde: Used in resins, disinfectants, and biological preservatives
- Benzaldehyde: Used in flavoring (almond scent), perfumes, and dyes
- Precursors in the synthesis of carboxylic acids and alcohols
Ketones
- Acetone: Common solvent in nail polish remover and laboratory settings
- Methyl ethyl ketone (MEK): Used in paint thinners and industrial cleaning agents
- Important intermediates in the production of plastics, pharmaceuticals, and perfumes
Biological Significance
- Aldehydes like retinal (derived from vitamin A) play a role in vision.
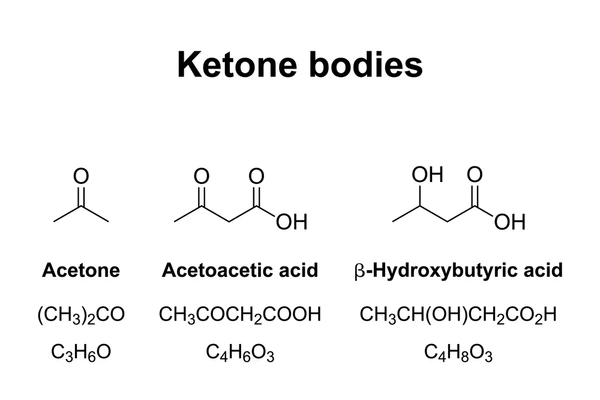
- Ketones such as acetoacetate and β-hydroxybutyrate are key energy sources during fasting (ketosis).
FAQs
Which is more reactive, aldehyde or ketone?
Aldehydes are generally more reactive because they have less steric hindrance and more electrophilic carbonyl carbons.
Can both aldehydes and ketones be oxidized?
Aldehydes can be oxidized to carboxylic acids easily. Ketones are more resistant and require strong oxidizers to break the carbon chain.
Do aldehydes and ketones undergo the same reactions?
They share some reaction types, like nucleophilic addition, but differ in oxidation and certain reagent-specific tests.
Why do ketones have higher boiling points than aldehydes?
The extra alkyl group in ketones increases van der Waals forces, leading to higher boiling points.
Are aldehydes or ketones more common in nature?
Both occur widely, but ketones are more stable and are often found in metabolic pathways and industrial products.
Conclusion
Aldehydes and ketones are central to organic chemistry, with similar functional groups but distinct structures and reactivity. Aldehydes feature a terminal carbonyl and are more prone to oxidation, while ketones have an internal carbonyl and are more stable. Both classes of compounds are essential in laboratory synthesis, industry, and biological systems. Understanding their differences helps chemists predict reactions, run identification tests, and design new molecules for various applications.
To get detailed scientific explanations of Aldehyde vs. Ketone, try Patsnap Eureka.


