
Aldol condensation is a reaction where two aldehydes or ketones, or a combination of both, react in the presence of a base or acid to form a β-hydroxy carbonyl compound (aldol), which then undergoes dehydration to yield an α,β-unsaturated carbonyl compound. The name “aldol” comes from the two functional groups involved: aldehyde + alcohol.
What is aldol condensation? Eureka Technical Q&A explains this key organic reaction where aldehydes or ketones combine to form larger β-hydroxy compounds, followed by dehydration, making it essential in synthetic chemistry and industrial applications.
General Reaction Format
Reactants: Aldehyde or ketone with α-hydrogen
Catalyst: Base (e.g., NaOH) or acid (e.g., HCl)
Product: α,β-unsaturated aldehyde or ketone
Overall Reaction (Base-catalyzed)
2 R–CH=O → R–CH(OH)–CH=O → R–CH=CH–CH=O (after dehydration)
Mechanism of Aldol Condensation
The aldol condensation mechanism typically begins with the deprotonation of a carbonyl compound to form an enolate ion or enol intermediate. This nucleophilic species then attacks another carbonyl compound, leading to the formation of a β-hydroxy aldehyde or ketone. Subsequent dehydration of this intermediate results in the formation of a conjugated enone .
1. Enolate Formation
In the presence of a base, the α-hydrogen of a carbonyl compound is removed to form an enolate ion.
2. Nucleophilic Attack
The enolate ion attacks the carbonyl carbon of another aldehyde or ketone molecule, forming a β-hydroxy aldehyde or ketone (aldol addition product).
3. Dehydration
Upon heating or with continued reaction conditions, the aldol product loses a water molecule, forming an α,β-unsaturated carbonyl compound.
This final product is stabilized by conjugation, making the reaction thermodynamically favorable.

Types of Aldol Condensation
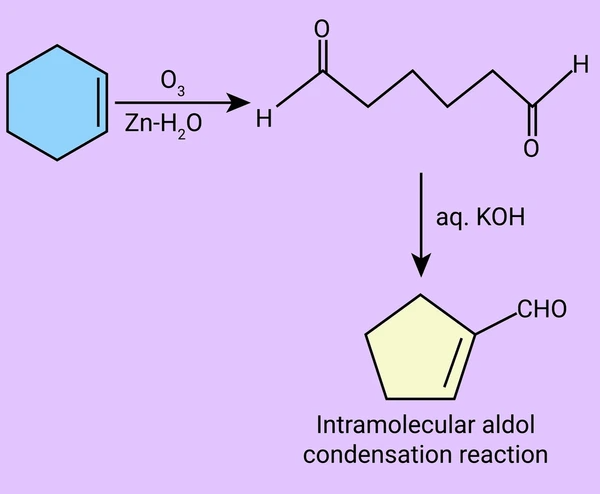
1. Intramolecular Aldol Condensation
A molecule with two carbonyl groups forms a ring via an intramolecular aldol reaction. This is common in the synthesis of five- or six-membered rings.
2. Crossed Aldol Condensation
Two different carbonyl compounds react together. It’s useful when one compound lacks α-hydrogens, minimizing multiple products.
3. Acid-Catalyzed Aldol Condensation
Although less common, acid can catalyze the formation of the enol instead of the enolate. The mechanism proceeds similarly but involves protonated intermediates.
Common Examples of Aldol Condensation
Acetaldehyde Self-Condensation
2 CH₃CHO → CH₃CH(OH)CH₂CHO → CH₃CH=CHCHO (crotonaldehyde)
This is a classic base-catalyzed aldol condensation forming an α,β-unsaturated aldehyde.
Acetone + Benzaldehyde (Crossed Aldol)
Acetone (with α-hydrogens) reacts with benzaldehyde (no α-hydrogens) to form cinnamaldehyde-like compounds used in perfumes and flavoring agents.
Intramolecular Example: 2,5-Hexanedione
Under basic conditions, 2,5-hexanedione cyclizes to form 3-methylcyclopentenone, a valuable compound in fragrance synthesis.
Applications of Aldol Condensation
Aldol condensation plays a vital role in modern chemistry. It enables the formation of carbon–carbon bonds and supports the development of fine chemicals, pharmaceuticals, and advanced materials. Here’s how industries use this powerful reaction.
Synthesis of Fine Chemicals
Chemists widely use this reaction to synthesize fine chemicals. The reaction combines aldehydes or ketones to form β-hydroxy aldehydes or ketones. These intermediates often undergo dehydration to produce α,β-unsaturated compounds. These unsaturated compounds serve as key building blocks in various high-value chemical products.
Pharmaceutical Applications
Pharmaceutical researchers rely on this reaction to build complex molecular structures with biological activity. For example, the reaction helps synthesize tricyclic compounds used in drug development. It also supports the creation of new medicinal agents with promising pharmacological properties.
Solvent and Industrial Chemical Production
Chemical manufacturers use this reaction to produce solvents like methyl isobutyl ketone (MIBK), often used in paints and coatings. It also enables the production of diacetone alcohol, mesityl oxide, and isophorone. These compounds serve as solvents and intermediates in adhesives, coatings, and polymer industries.
Organic Synthesis
In organic chemistry, aldol condensation remains a crucial method for forming carbon–carbon bonds. Chemists use it to build complex molecules through multistep reactions, including dehydration and further functionalization.
Biocatalysis and Green Chemistry
Recent advances focus on using biocatalysts and organocatalysts for more sustainable aldol reactions. These methods often use water as a solvent and generate fewer byproducts. As a result, researchers can reduce environmental impact and improve reaction efficiency.
Material Science Applications
Engineers use this reaction to synthesize materials for organic thin-film transistors and other electronic devices. It helps create compounds with specific electronic properties needed in next-generation materials.
Factors Affecting Aldol Reactions
- Base or Acid Strength: Influences enolate or enol formation
- Temperature: Higher temperatures favor dehydration
- Sterics and Electronics: Bulky substituents or electron-withdrawing groups affect product formation
- Presence of α-Hydrogens: Only carbonyl compounds with α-hydrogens can form enolates
FAQs
Why is dehydration favored in aldol condensation?
Dehydration forms a conjugated double bond system (α,β-unsaturated carbonyl), which is more stable and lowers the overall energy.
Can ketones undergo aldol condensation?
Yes, although aldehydes are generally more reactive. Ketones need stronger conditions or longer reaction times.
Is aldol condensation reversible?
The initial aldol addition is reversible, but the dehydration step usually drives the reaction forward irreversibly under heat.
What’s the difference between aldol addition and aldol condensation?
Aldol addition stops at the β-hydroxy carbonyl stage. Condensation includes the dehydration step to form the unsaturated product.
Can you perform aldol condensation in water?
Yes, it often occurs in aqueous base (e.g., NaOH) under room temperature or mild heating conditions.
Conclusion
Aldol condensation is a cornerstone of organic synthesis, enabling chemists to build larger, more complex molecules efficiently. From pharmaceuticals to materials science, this reaction forms the basis of many practical applications. By understanding its mechanism, types, and factors that influence its outcome, chemists can apply it strategically in both academic and industrial settings.
To get detailed scientific explanations of Aldol Condensation, try Patsnap Eureka.


