
Artificial Pancreas Evolution and Objectives
The concept of an artificial pancreas has evolved significantly over the past few decades, driven by the growing need for more effective management of diabetes. This technological innovation aims to mimic the function of a healthy pancreas by automatically controlling blood glucose levels in individuals with diabetes. The journey of artificial pancreas development began in the 1960s with the introduction of continuous glucose monitoring (CGM) systems, which laid the foundation for more advanced closed-loop systems.
If you’re navigating the complexities of artificial pancreas systems—whether for research, development, or clinical insight—Eureka Technical Q&A connects you with medical technology experts who can break down design principles, regulatory hurdles, and integration challenges in real time.
The primary objective of artificial pancreas technology is to create a fully automated system that can monitor blood glucose levels, calculate the appropriate insulin dose, and deliver it without human intervention. This ambitious goal seeks to alleviate the burden of constant self-management for people with diabetes and reduce the risk of complications associated with poor glycemic control. As the technology has progressed, intermediate objectives have emerged, such as the development of hybrid closed-loop systems that require some user input but offer significant improvements in glucose control.
The evolution of artificial pancreas technology has been marked by several key milestones. In the 1970s and 1980s, researchers began exploring the possibility of using computer algorithms to control insulin delivery based on glucose readings. The 1990s saw the development of more sophisticated CGM devices and insulin pumps, paving the way for the first closed-loop systems. In 2016, the FDA approved the first hybrid closed-loop system, marking a significant step towards a fully automated artificial pancreas.
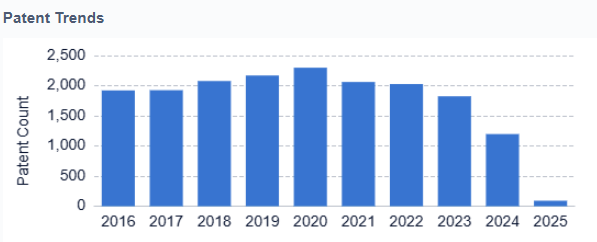
Current objectives in artificial pancreas development include improving the accuracy and reliability of glucose sensors, enhancing the responsiveness of insulin delivery systems, and refining control algorithms to better predict and respond to changes in blood glucose levels. Researchers are also focusing on developing more user-friendly interfaces and integrating additional features such as exercise and meal detection to create more comprehensive and adaptable systems.
Looking ahead, the ultimate goal is to create a “smart” artificial pancreas that can learn from individual patient data and adapt its insulin delivery strategy accordingly. This would involve incorporating advanced machine learning algorithms and potentially integrating additional hormone delivery, such as glucagon, to more closely mimic the function of a biological pancreas. Additionally, there is a push towards miniaturization and improved wearability to enhance user comfort and adherence.
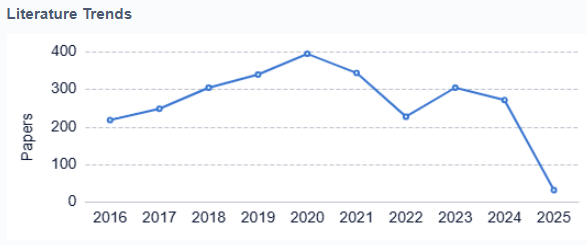
As the field continues to advance, researchers and developers are also addressing challenges such as cybersecurity, interoperability between different devices and systems, and regulatory approval processes. The ongoing evolution of artificial pancreas technology holds great promise for improving the quality of life for millions of people with diabetes worldwide, with the potential to revolutionize diabetes management in the coming years.
Diabetes Management Market Analysis
The diabetes management market has been experiencing significant growth and transformation in recent years, driven by the increasing prevalence of diabetes worldwide and the growing demand for more effective and convenient treatment options. The global diabetes management market was valued at approximately $58.5 billion in 2020 and is projected to reach $78.3 billion by 2026, growing at a CAGR of 5.2% during the forecast period. This growth is primarily attributed to the rising diabetic population, technological advancements in diabetes care devices, and increasing awareness about diabetes management.
The market is segmented into various categories, including blood glucose monitoring devices, insulin delivery devices, and diabetes management software. Among these, the blood glucose monitoring devices segment currently holds the largest market share, accounting for about 40% of the total market value. However, the insulin delivery devices segment, which includes artificial pancreas systems, is expected to witness the highest growth rate in the coming years.
The artificial pancreas market, a subset of the broader diabetes management market, is experiencing rapid expansion. It is estimated to grow from $123.5 million in 2020 to $451.8 million by 2027, with a CAGR of 20.3%. This growth is driven by the increasing adoption of closed-loop systems, advancements in continuous glucose monitoring (CGM) technology, and the rising demand for automated insulin delivery systems.
Key market trends in diabetes management include the shift towards personalized medicine, the integration of artificial intelligence and machine learning in diabetes care devices, and the growing popularity of wearable and connected devices. Additionally, there is a rising focus on developing all-in-one diabetes management solutions that combine glucose monitoring, insulin delivery, and data analytics.
The market is highly competitive, with major players such as Medtronic, Dexcom, Abbott Laboratories, and Insulet Corporation dominating the space. These companies are continuously investing in research and development to improve their product offerings and gain a competitive edge. Moreover, several startups and emerging companies are entering the market with innovative solutions, further intensifying the competition.
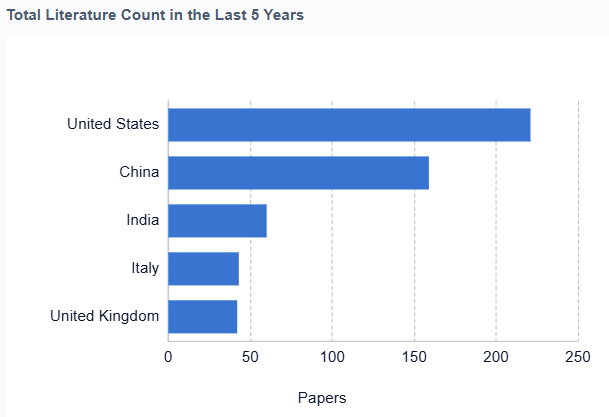
Geographically, North America currently leads the diabetes management market, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years due to the increasing diabetic population, rising healthcare expenditure, and growing awareness about diabetes management in countries like China and India.
In conclusion, the diabetes management market, particularly the artificial pancreas segment, presents significant growth opportunities driven by technological advancements and increasing demand for automated and personalized diabetes care solutions. As the market continues to evolve, companies that can offer innovative, user-friendly, and cost-effective solutions are likely to gain a competitive advantage and capture a larger market share.
Technical Hurdles in Artificial Pancreas Development
The artificial pancreas is a promising solution for managing type 1 diabetes. However, its development still faces several technical challenges that must be addressed before widespread adoption.
1. Sensor Accuracy and Reliability
Continuous glucose monitors (CGMs) are central to artificial pancreas systems. These sensors must provide accurate, real-time glucose readings. Even minor errors can lead to incorrect insulin dosing. Researchers are working to improve sensor calibration, reduce lag time, and enhance reliability during daily activities.
2. Insulin Delivery Delays
Insulin pumps can’t always match the body’s natural insulin response. Delayed insulin absorption, especially with subcutaneous delivery, makes it difficult to control blood sugar spikes. Faster-acting insulins and alternative delivery methods like intradermal or inhalable insulin are under development.

3. Algorithm Precision
Control algorithms must predict glucose changes and adjust insulin delivery accurately. Developing models that handle meals, exercise, stress, and sleep patterns in real time remains a challenge. Machine learning is helping improve these systems, but ensuring safety remains a top priority.
4. Integration and Communication
Artificial pancreas systems require seamless communication between sensors, insulin pumps, and control algorithms. Technical glitches or Bluetooth failures can disrupt the system’s performance. Developers aim to build more robust and secure wireless communication channels.
5. Power and Portability
Users need compact, lightweight systems that offer long battery life. Current devices can still be bulky or require frequent charging. Engineers are exploring low-power electronics and wireless charging solutions to improve usability.
6. Personalization and Adaptability
No two patients are the same. Artificial pancreas systems must adapt to each person’s unique metabolism and lifestyle. Creating adaptive systems that learn from user behavior is key to long-term success.
7. Cost and Accessibility
Even the most advanced systems must remain affordable and accessible. High production costs and limited insurance coverage create barriers for many patients. Lowering manufacturing costs and expanding reimbursement options are vital for broader adoption.
8. Regulatory and Safety Requirements
These systems must meet strict regulatory standards to ensure patient safety. Proving long-term reliability, managing risks, and obtaining FDA or international approvals can delay product launches. Ongoing clinical trials aim to provide the data needed to move forward.
Glucose Monitoring Technology Progression
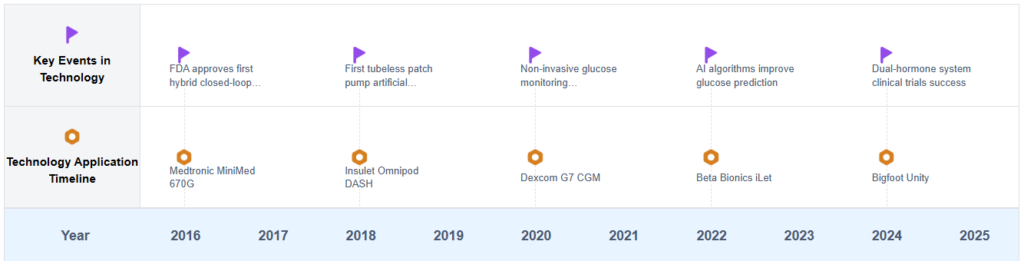
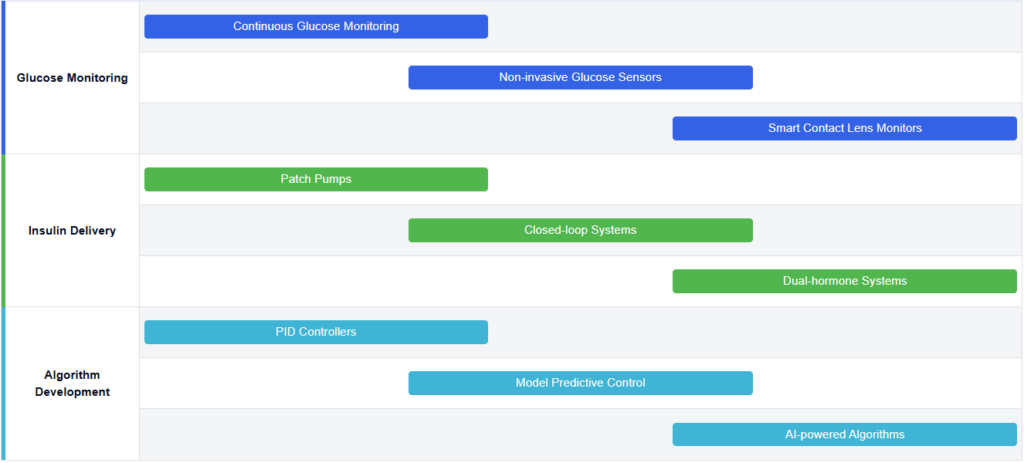
Key Artificial Pancreas Developers
The artificial pancreas market is in a growth phase, driven by increasing diabetes prevalence and technological advancements. The market size is expanding rapidly, with projections indicating significant growth in the coming years. Technologically, the field is progressing but still faces challenges in achieving fully automated, closed-loop systems. Companies like Medtronic, Insulet, and DexCom are at the forefront, developing advanced algorithms and sensor technologies. Emerging players such as Diabeloop and Ypsomed are also making strides. Academic institutions like Harvard, University of California, and Arizona State University contribute significantly to research and innovation. The competitive landscape is characterized by a mix of established medical device companies and innovative startups, all working towards more accurate, user-friendly, and integrated artificial pancreas solutions.
 Medtrum Technologies, Inc.
Medtrum Technologies, Inc.
Technical Solution
Medtrum’s A7+ TouchCare System is an advanced artificial pancreas solution that includes a patch pump, continuous glucose monitor, and a smartphone app for control. The system uses a predictive low glucose suspend algorithm to prevent hypoglycemia and can automatically adjust basal insulin rates. It features a unique touch-screen patch pump design, allowing for discreet insulin delivery without the need for a separate controller device.
Strengths: Integrated patch pump design improves user convenience. Touch-screen interface enhances usability.
Weaknesses: Limited market presence compared to larger competitors, which may affect user trust and adoption.
 Eli Lilly & Co.
Eli Lilly & Co.
Technical Solution
Eli Lilly is developing an automated insulin delivery system that combines a hybrid closed-loop insulin pump with a continuous glucose monitor. Their system uses a proprietary algorithm to automatically adjust insulin delivery based on glucose readings. Additionally, Eli Lilly is working on smart insulin pens and connected insulin management systems to improve diabetes care. Their approach focuses on integrating various diabetes management tools into a cohesive ecosystem.
Strengths: Comprehensive diabetes management ecosystem. Leverages extensive pharmaceutical expertise.
Weaknesses: Relatively new entrant in the artificial pancreas market compared to established device manufacturers.
 Insulet Corp.
Insulet Corp.
Technical Solution
Insulet’s Omnipod 5 Automated Insulin Delivery System is a tubeless, wearable artificial pancreas solution. It uses a continuous glucose monitor to track blood sugar levels and automatically adjusts insulin delivery through a pod adhered to the body. The system employs an adaptive algorithm that learns the user’s insulin needs over time, providing personalized insulin delivery. It also features a smartphone app for remote monitoring and control.
Strengths: Tubeless design improves user comfort and convenience. Adaptive algorithm enhances personalized treatment.
Weaknesses: Requires regular pod changes and may have adhesive-related skin issues for some users.
 Diabeloop SAS
Diabeloop SAS
Technical Solution
Diabeloop’s DBLG1 System is an advanced artificial pancreas solution that combines a continuous glucose monitor, an insulin pump, and a dedicated handset running their proprietary self-learning algorithm. The system analyzes real-time data to automatically adjust insulin delivery, considering factors such as meal intake, physical activity, and sleep patterns. It also features a user-friendly interface for manual interventions and data visualization.
Strengths: Comprehensive system with advanced self-learning algorithm. Considers multiple factors for insulin adjustment.
Weaknesses: May have a steeper learning curve for users due to its complexity.
 DexCom, Inc.
DexCom, Inc.
Technical Solution
DexCom’s G7 Continuous Glucose Monitoring (CGM) system is a key component in artificial pancreas solutions. It provides real-time glucose readings every 5 minutes, with exceptional accuracy and reliability. The system includes a small, wearable sensor and transmitter, sending data to a smartphone app or dedicated receiver. DexCom’s CGM integrates with various insulin delivery systems, forming a crucial part of closed-loop artificial pancreas systems.
Strengths: High accuracy and reliability in glucose monitoring. Seamless integration with multiple insulin delivery systems.
Weaknesses: Requires sensor replacement every 10 days, which may be inconvenient for some users.
Current Artificial Pancreas Solutions
Glucose monitoring and insulin delivery systems
- Artificial pancreas systems integrate continuous glucose monitoring with automated insulin delivery. These closed-loop systems aim to mimic the function of a healthy pancreas by adjusting insulin doses based on real-time glucose readings, improving glycemic control for diabetic patients.
- Integrated glucose monitoring and insulin delivery systems
These systems combine continuous glucose monitoring with automated insulin delivery, forming the basis of an artificial pancreas. They use algorithms to analyze real-time glucose data and adjust insulin delivery accordingly, mimicking the function of a healthy pancreas. This approach aims to improve glycemic control and reduce the burden of diabetes management for patients. - Advanced algorithms for glucose prediction and insulin dosing
Sophisticated algorithms are developed to predict future glucose levels based on current trends, meal intake, and other factors. These predictive models enable more accurate and timely insulin dosing decisions, potentially preventing hypoglycemic and hyperglycemic events. Machine learning and artificial intelligence techniques are often employed to enhance the accuracy and personalization of these algorithms. - Wearable and implantable devices for continuous monitoring
Innovative wearable and implantable devices are designed for continuous glucose monitoring, offering improved accuracy, longer sensor life, and enhanced user comfort. These devices may incorporate advanced sensing technologies, wireless communication capabilities, and extended battery life to provide seamless, long-term glucose monitoring for artificial pancreas systems. - Closed-loop systems with adaptive control
Advanced closed-loop systems incorporate adaptive control mechanisms that learn from an individual’s glucose patterns and insulin responses over time. These systems can adjust their control parameters automatically to optimize glucose management for each user, accounting for variations in insulin sensitivity, physical activity, and other factors that influence glucose levels.
Algorithm development for insulin dosing
Advanced algorithms are crucial for artificial pancreas systems to determine optimal insulin dosing. These algorithms process glucose data, predict future trends, and calculate appropriate insulin delivery rates, considering factors such as meal intake, physical activity, and individual patient characteristics.
Integration of additional sensors and data sources
Artificial pancreas systems are being enhanced by incorporating additional sensors and data sources beyond glucose monitoring. This may include activity trackers, heart rate monitors, or other physiological sensors to provide a more comprehensive view of the patient’s metabolic state and improve insulin dosing accuracy.
Implantable artificial pancreas devices
Research is ongoing to develop fully implantable artificial pancreas systems. These devices aim to provide long-term, autonomous glucose regulation with minimal external intervention, potentially offering improved quality of life for diabetic patients.
Bioengineered pancreatic cells and tissues
Advancements in bioengineering and stem cell research are exploring the creation of artificial pancreatic tissues or cells. These approaches aim to develop biological alternatives to electronic artificial pancreas systems, potentially offering more physiological glucose regulation.
Innovative Glucose Control Algorithms
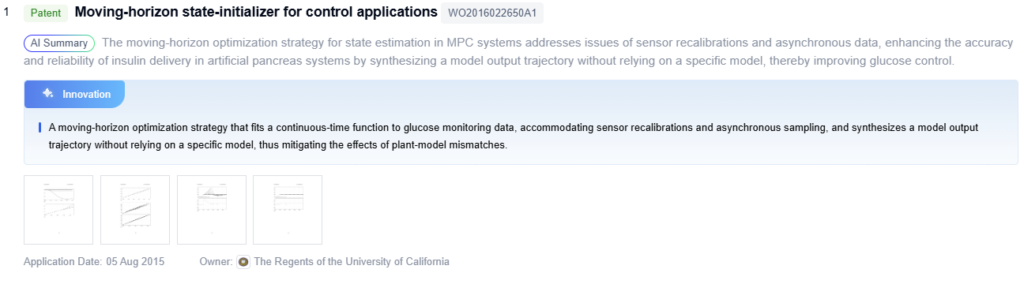

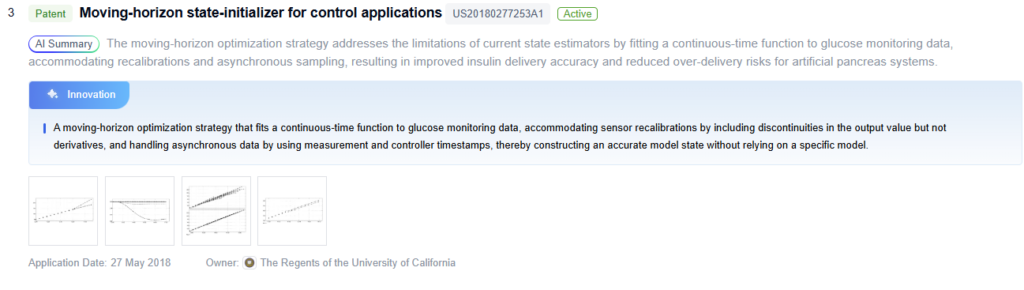
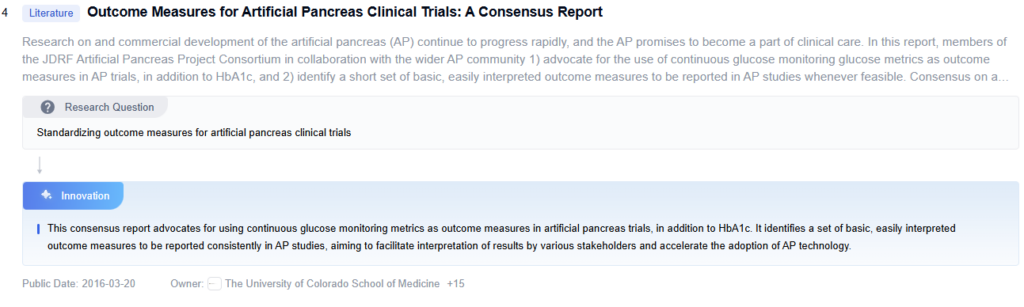
Future Artificial Pancreas Advancements
Closed-Loop AI-Driven Artificial Pancreas
The closed-loop AI-driven artificial pancreas is revolutionizing how patients manage type 1 diabetes. By combining continuous glucose monitoring (CGM), an insulin pump, and artificial intelligence, this system delivers fully automated insulin control.
How the System Works
At the heart of this technology is an AI engine that processes real-time glucose readings. It uses machine learning to analyze past trends and current physiological data. Based on this, it predicts blood sugar changes and adjusts insulin delivery automatically.
The system considers multiple factors, including meals, activity levels, stress, and sleep. This helps it mimic how a healthy pancreas would respond. Over time, the algorithm learns from each user’s unique patterns, offering a more personalized and adaptive insulin strategy.
Handling Complex Scenarios
Traditional pumps struggle with unpredictable blood sugar swings. This AI-driven system handles those better. It can manage challenges like the dawn phenomenon or post-exercise glucose drops. The AI detects patterns and adjusts insulin in advance to prevent dangerous highs or lows.
Built-In Safety Features
The system includes smart safety functions like predictive low-glucose suspend and automated correction boluses. These features reduce the risk of hypoglycemia and limit the need for manual intervention. As a result, users gain tighter glucose control with less daily stress.
Smart Connectivity and Remote Monitoring
Integration with smartphones and cloud platforms enhances its usability. Patients can monitor glucose levels, share data with caregivers, and receive support from healthcare providers in real time. This connectivity also helps doctors make informed adjustments to treatment plans.
Future Enhancements
Researchers are working on adding more sensors to the system. These may track ketones, cortisol, or other hormones that affect blood sugar. With more data, the system could make even smarter insulin decisions, further improving health outcomes.
Key Strengths
- Delivers adaptive and highly personalized insulin dosing
- Reduces manual intervention and daily management burden
- Enhances quality of life with better glucose control
- Connects to digital health tools for coordinated care
Notable Weaknesses
- High initial cost and possible insurance limitations
- System depends on reliable tech and continuous connectivity
- Raises cybersecurity concerns for patient data
- Faces strict regulatory approval for AI-driven medical devices
Regulatory Framework for Medical Devices
The regulatory framework for medical devices plays a vital role in the safe development and approval of artificial pancreas systems. These advanced systems, designed to automate insulin delivery and glucose monitoring, are classified as high-risk medical devices due to their critical function in managing type 1 diabetes.
In the United States, the Food and Drug Administration (FDA) oversees the approval process for artificial pancreas systems. The FDA classifies them as Class III devices, which requires a rigorous premarket approval (PMA) process. Manufacturers must provide clinical trial data to demonstrate the safety and effectiveness of the device. The FDA has also issued specific guidelines for hybrid and closed-loop systems to ensure that these technologies meet high standards before entering the market.
In the European Union, the Medical Device Regulation (MDR), which came into effect in 2021, sets the framework for approving artificial pancreas systems. These devices are generally classified as Class III under the MDR as well. To bring a product to market, manufacturers must undergo a conformity assessment by a notified body, submit clinical evidence, and obtain a CE mark. This approval allows the device to be sold across EU member states.
Other countries, such as Japan and China, have also developed specific regulatory pathways for artificial pancreas systems. While these systems often mirror the regulatory models established by the FDA and the EU, they include unique requirements tailored to their healthcare systems. These may involve national trials, performance testing, and compliance with local safety regulations.
Post-market surveillance is another critical component of the regulatory framework. Once a device is approved and in use, manufacturers are responsible for continuous monitoring to ensure ongoing safety and performance. This includes maintaining a quality management system and implementing a vigilance program to identify and respond to any adverse events or product failures.
As artificial pancreas systems become increasingly connected to digital platforms, regulators are placing more focus on interoperability and cybersecurity. Devices must be able to communicate safely with smartphones, apps, and cloud services without risking data breaches or functional disruptions. Regulatory agencies are developing specific guidelines to address these issues.
To keep pace with rapid technological advancements, regulatory bodies are also adopting more flexible approval processes. Accelerated pathways are now available for breakthrough medical devices, allowing for faster evaluation without compromising safety. Additionally, real-world evidence, including patient data collected from wearable devices, is playing a growing role in regulatory decision-making.
Ultimately, the success of artificial pancreas regulation depends on collaboration between industry, regulators, and healthcare providers. Open communication helps create a regulatory environment that prioritizes patient safety while supporting innovation. As artificial pancreas technology continues to evolve, the regulatory framework must remain responsive and adaptive to ensure safe and timely access for those who need it most.
Patient Adoption and User Experience
Patient adoption and user experience are essential to the success of artificial pancreas systems. As these devices become more advanced and widely available, understanding how patients interact with them helps improve usability and encourages broader adoption.
Early research shows encouraging results. Many patients report better blood sugar control and reduced stress from diabetes management. However, challenges still exist. Users often struggle with complex interfaces, discomfort from wearing the devices, and the steep learning curve that comes with new technology. Concerns about system reliability, technical glitches, and the need for constant monitoring are also common.
Feedback from user experience surveys highlights areas for improvement. Most patients appreciate the benefits of automated insulin delivery and continuous glucose monitoring. However, frequent alarms and alerts can interrupt daily routines and cause frustration. Many users want more intuitive interfaces and flexible settings that align with their personal habits and preferences.
The psychological side of adopting an artificial pancreas is also important. While many feel more secure using the technology, others find it difficult to hand over control to an automated system. This can lead to anxiety or resistance, especially in the early stages of use. To ease the transition, educational programs and user support are critical. These resources can help patients feel confident and capable when using their devices.
Ongoing long-term studies continue to assess user satisfaction and adherence. So far, findings suggest that despite early challenges, most patients find the benefits worth the adjustment. As technology improves, manufacturers are using patient feedback to design more comfortable, user-friendly systems that better integrate into everyday life.
Patients increasingly value the ability to connect artificial pancreas systems with smartphones and digital health platforms. Being able to track progress, adjust settings, and share data with healthcare providers improves both convenience and quality of care. This interoperability is becoming a must-have feature for many users.
As the market for artificial pancreas systems grows, patient advocacy groups are influencing development and regulation. Their input ensures that new devices are not only technically advanced but also meet the real needs of the people who rely on them daily.
In conclusion, while most patients have positive experiences with artificial pancreas systems, there is still room for improvement. Developers must continue listening to users and refining features to build trust, comfort, and long-term success in managing diabetes with these life-changing tools.
To get detailed scientific explanations of Artificial Pancreas, try Patsnap Eureka.


