
Beer’s Law (also known as the Beer-Lambert Law) is a fundamental principle in chemistry and physics that describes how light absorption relates to the concentration of a substance in a solution. This law plays a crucial role in spectrophotometry and serves as a vital tool in fields like analytical chemistry, biochemistry, environmental science, and pharmaceuticals for measuring solute concentrations. In this article, we will explore the definition, formula, key applications, and practical examples of Beer’s Law to enhance your understanding of its significance in scientific research and industry.
What is Beer’s Law?
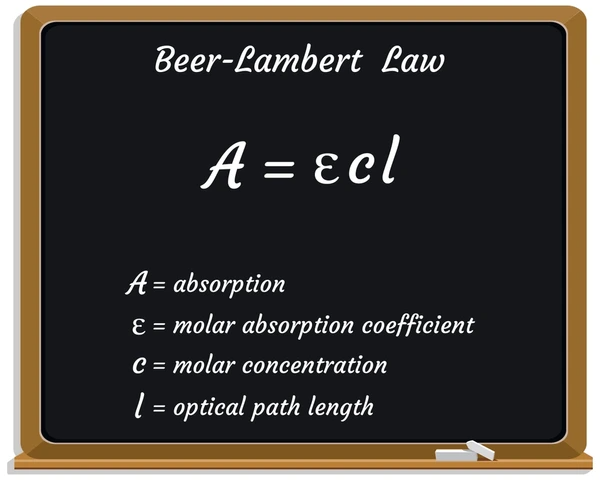
Beer’s Law establishes a linear relationship between absorbance (A), concentration (c), path length (l), and the molar absorptivity (ε) of a substance. This law allows scientists to calculate the concentration of a substance in solution by measuring how much light the substance absorbs at a specific wavelength.
Beer’s Law Formula
Key Concepts in Beer’s Law
Absorbance
Absorbance refers to the amount of light a solution absorbs at a particular wavelength. A higher absorbance indicates that the substance absorbs more light, suggesting a higher concentration of the solute.
Molar Absorptivity (ε)
Molar absorptivity, or molar absorption coefficient, measures how strongly a substance absorbs light at a given wavelength. The molar absorptivity varies by substance and wavelength. Substances with higher molar absorptivity absorb more light.
Path Length (l)
Path length refers to the distance the light travels through the sample. In most cases, a standard 1 cm path length is used, though it can vary depending on the experiment.
Concentration (c)
The concentration of a solute in a solution directly influences the absorbance. As the concentration increases, the absorbance increases, assuming the substance obeys Beer-Lambert Law.
Applications of Beer’s Law
💡 Curious about how Beer’s Law applies in real-world scenarios? Eureka Technical Q&A offers expert insights into its use in analytical chemistry, environmental monitoring, and medical diagnostics, helping you understand how this fundamental principle can enhance your research and applications.
1. Concentration Determination
Scientists commonly use Beer-Lambert Law to determine the concentration of a solute. By measuring the absorbance at a specific wavelength and knowing the molar absorptivity and path length, they can calculate the concentration. This method is frequently applied in chemical analysis, biochemistry, and pharmaceuticals.
Example:
In a lab, scientists dissolve copper sulfate in a solution. By measuring the absorbance at a specific wavelength, they can apply Beer’s Law to calculate the concentration of copper sulfate.
2. Environmental Monitoring
Beer’s Law is essential in environmental science for measuring the concentration of pollutants in water, air, and soil. For example, scientists use Beer-Lambert Lawto measure the nitrate concentrations in water samples by analyzing the absorbance at a specific wavelength.
Example:
In a wastewater treatment plant, technicians measure nitrates and ammonia concentrations using spectrophotometric methods. They apply Beer-Lambert Law to convert absorbance readings into pollutant concentrations.
3. Pharmaceutical Industry
Beer’s Law is also widely used in the pharmaceutical industry to determine the concentration of drugs in solutions. Manufacturers use it for quality control during production and for analyzing biological samples.
Example:
Scientists measure the vitamin C content in oral tablets by dissolving the tablets and measuring the absorbance at a specific wavelength. They apply Beer-Lambert Law to calculate the concentration of vitamin C in the tablets.
4. Food and Beverage Industry
In food science, Beer’s Law helps measure the concentration of coloring agents, sugars, and additives in beverages and food products. Food scientists use spectrophotometry to ensure that products meet regulatory standards.
Example:
Scientists measure anthocyanins in fruit juices using Beer-Lambert Law. This ensures that the color intensity remains consistent and meets quality standards.
Application Cases
| Product/Project | Technical Outcomes | Application Scenarios |
|---|---|---|
| Variable Path Length Photon Trapping Spectrometer Uwm Research Foundation, Inc. | Increases effective path lengths using rotating reflector and slit arrangement, enabling detection of low concentration samples | High-precision spectroscopy for trace analysis in environmental and chemical research |
| Linear Optical Loss Probe Finesse Solutions, Inc. | Uses monochromatic light source and phase-sensitive detection with apertured solid angle limitation for accurate cell density monitoring | Real-time monitoring of cell density in bioreactors for biotechnology and pharmaceutical applications |
| OPS Imaging System Correction Method Cytometrics, Inc. | Improves accuracy of hemoglobin concentration measurements by estimating and subtracting scattered light | Intravital microscopy for medical diagnostics and research in blood-related disorders |
| Plasmonic Nanoantenna Infrared Absorption Spectroscopy École Polytechnique Fédérale de Lausanne | Enables sensitive, real-time infrared absorption spectroscopy of biomolecules in aqueous environments, overcoming water absorption issues | Biomolecular analysis in pharmaceutical research and development, and medical diagnostics |
| Depth-Resolved Reflectance Instrument Wisconsin Alumni Research Foundation | Utilizes Monte Carlo-based models for accurate measurement of optical properties in layered epithelial tissues | Enhanced diagnostic capabilities for epithelial pre-cancers and cancers in medical imaging |
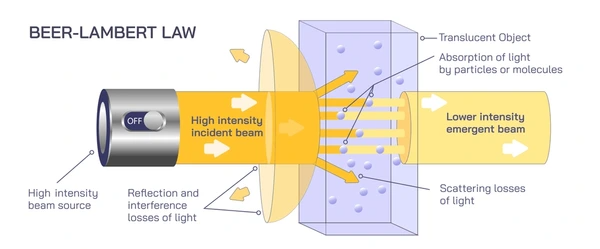
Key Assumptions and Limitations of Beer’s Law
Assumptions:
- Linear Relationship: Beer’s Law assumes a linear relationship between absorbance and concentration. This linearity holds true for dilute solutions where chemical interactions do not affect absorbance.
- Monochromatic Light: The law assumes the light used is monochromatic, meaning it has a single wavelength.
- Homogeneous Solution: The solution must be homogeneous, meaning the solute should be evenly distributed throughout the solvent.
- No Chemical Interactions: Beer-Lambert Law assumes no chemical reactions alter the absorption characteristics of the solute.
Limitations:
- High Concentrations: At high concentrations, deviations from linearity may occur due to instrumental limitations or chemical interactions between solutes and solvents.
- Scattering Effects: Beer’s Law assumes that all light is absorbed, but solutions with suspended particles or turbidity can scatter light.
- Wavelength Dependency: Accurate application of Beer-Lambert Law requires selecting the correct wavelength, as different substances absorb light at different wavelengths.
Beer’s Law in Action: Real-World Example
Case Study: Determining Glucose Levels in Blood Using Beer-Lambert Law
In medical diagnostics, Beer’s Law plays a key role in measuring blood glucose levels. During testing, glucose oxidase catalyzes a reaction where glucose reacts with oxygen, producing a colored product. The absorbance of this product correlates with the glucose concentration. Technicians apply Beer-Lambert Law to calculate the glucose levels in blood samples.
Conclusion
Beer’s Law is a fundamental principle in analytical chemistry and spectroscopy. It offers a simple way to determine solute concentrations based on light absorption. Understanding the law’s formula, assumptions, and limitations allows researchers and scientists to apply it across various fields, including environmental science, pharmaceuticals, food science, and medical diagnostics. Beer-Lambert Law continues to be a critical tool in both laboratory and industrial applications, providing insights into the nature of solute concentration and light absorption.
FAQs
1. How is Beer-Lambert Law used in environmental science?
Scientists use Beer’s Law to measure pollutant concentrations in environmental samples, such as water and soil, by analyzing absorbance at specific wavelengths.
2. Can Beer-Lambert Law be used at any concentration?
Beer’s Law is valid for dilute solutions. For concentrated solutions, deviations from linearity may occur due to chemical interactions or limitations of the spectrophotometer.
3. Why is wavelength important in Beer-Lambert Law?
The absorption of light varies with wavelength, so selecting the correct wavelength is critical. Accurate measurements depend on using the wavelength where the substance absorbs light most strongly.
To get detailed scientific explanations of Beer’s Law, try Patsnap Eureka.


