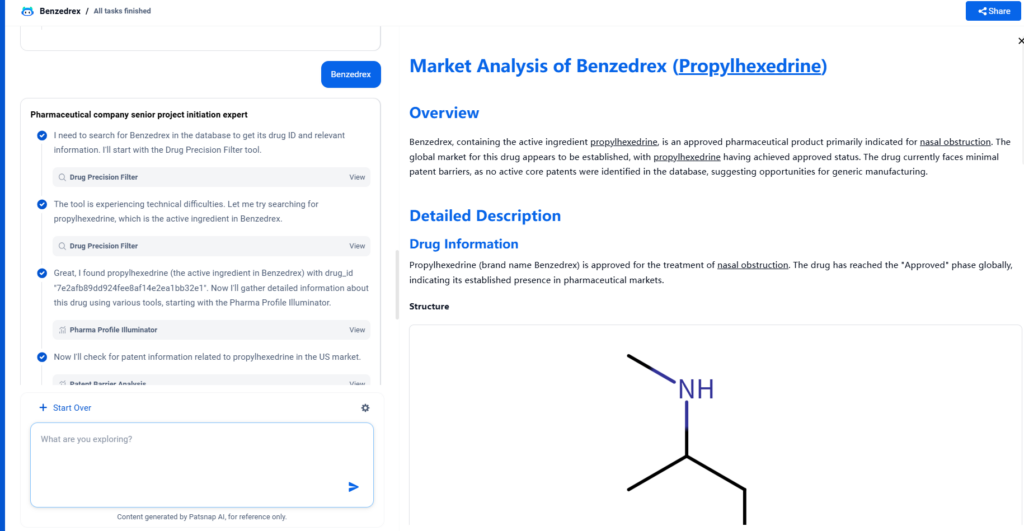
Overview
Benzedrex, containing the active ingredient propylhexedrine, is an approved pharmaceutical product primarily indicated for nasal obstruction. The global market for this drug appears to be established, with propylhexedrine having achieved approved status. The drug currently faces minimal patent barriers, as no active core patents were identified in the database, suggesting opportunities for generic manufacturing.
Detailed Description
Drug Information
Propylhexedrine (brand name Benzedrex) is approved for the treatment of nasal obstruction. The drug has reached the “Approved” phase globally, indicating its established presence in pharmaceutical markets.
Patent Barrier Analysis
Registration Patent Analysis
No registration patents were identified for propylhexedrine in the US market. This suggests that the original patents protecting the compound have expired, removing the primary intellectual property barriers to market entry.
Other Patent Barrier Analysis
Only one non-original patent was identified related to propylhexedrine:
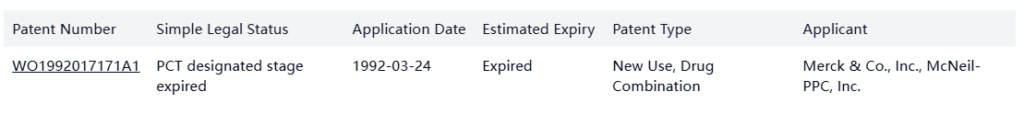
This patent covered “Ibuprofen-decongestant combinations” and has expired, removing any patent barriers for these specific combinations. The absence of active patents suggests that propylhexedrine can be manufactured and marketed without significant intellectual property constraints.
Clinical Results
The FDA Label Clinical Insight reveals important clinical information about propylhexedrine:
- Toxicological and Forensic Investigations:
- Studies have documented cases of propylhexedrine abuse and associated fatalities dating back to the 1970s.
- Autopsy findings in 15 individuals with intravenous propylhexedrine toxicity revealed anatomical changes including right ventricular hypertrophy and signs of pulmonary hypertension, interpreted as direct toxic effects of the drug .
- Case Report and Literature Reviews:
- Recent publications have examined the resurgence of propylhexedrine abuse by compiling toxicological data and clinical presentations.
- Documented adverse effects include headache, tremor, chest pain, arrhythmias (palpitations and tachycardia), psychosis, and pulmonary edema .
- These studies relied on clinical case analyses, patient histories, and post-mortem examinations to correlate symptoms with documented propylhexedrine exposure.
- Analytical Methodologies:
- Forensic investigations likely utilized techniques such as gas chromatography–mass spectrometry (GC-MS) to quantify propylhexedrine in biological specimens, though specific measurement protocols were not detailed in the available summaries .
Infringement Cases
No patent infringement incidents involving propylhexedrine were identified in the database 3. This further supports the conclusion that there are minimal intellectual property barriers to manufacturing and marketing propylhexedrine-based products.
Policy and Regulatory Risk Warning
Based on the available information, there appear to be no identified market exclusivity periods or data protection terms for propylhexedrine in the US market. However, given the documented abuse potential and toxicity concerns, regulatory oversight of propylhexedrine products may be significant despite the absence of patent protection.
Market Entry Assessment & Recommendations
- Generic Manufacturing Opportunity: With no active patent protection identified, propylhexedrine presents an opportunity for generic pharmaceutical manufacturers to enter the market with minimal intellectual property constraints.
- Safety Profile Considerations: The significant toxicological concerns and documented abuse potential of propylhexedrine necessitate careful consideration of risk management strategies. Any market entry plan should include robust safety monitoring and clear patient education materials.
- Formulation Innovation: While the base compound appears to be off-patent, there may be opportunities for developing improved formulations with enhanced safety profiles or novel delivery systems that could qualify for new patent protection.
- Regulatory Strategy: Despite the absence of patent barriers, companies should engage proactively with regulatory authorities regarding the known safety concerns associated with propylhexedrine. This may involve developing Risk Evaluation and Mitigation Strategies (REMS) or other safety programs.
- Market Differentiation: Given the mature status of propylhexedrine in the market, companies should consider strategies to differentiate their products, such as combination therapies (with non-protected compounds), improved formulations, or targeted marketing approaches.
- Abuse Deterrence: In light of the documented abuse potential, developing abuse-deterrent formulations could provide both a competitive advantage and address an important public health concern.
- Market Education: There appears to be a need for healthcare provider and patient education regarding the appropriate use and risks associated with propylhexedrine, which could be integrated into marketing and post-marketing strategies.
The absence of patent protection, combined with the established clinical use of propylhexedrine, suggests that the market for Benzedrex and related products may be accessible to generic manufacturers and companies seeking to develop improved formulations of this nasal decongestant.
Looking to explore off-patent drug opportunities like Benzedrex? PatSnap Eureka AI Agent can help you assess risk, IP barriers, and launch timelines with confidence.