
BiVacor TAH Background and Objectives
The BiVacor Total Artificial Heart (TAH) represents a groundbreaking advancement in the field of cardiovascular medical devices, aiming to provide a long-term solution for patients with end-stage biventricular heart failure. This innovative technology has emerged from decades of research and development in artificial heart systems, building upon the limitations of previous designs to offer a more sustainable and effective alternative. The BiVacor TAH is designed to completely replace the native heart, providing full circulatory support to patients who are not candidates for heart transplantation or as a bridge to transplantation.
Curious about how the BiVacor Total Artificial Heart works or its underlying engineering? Eureka Technical Q&A connects you with real experts who break down complex biomedical innovations, offering fast, reliable insights that help you understand cutting-edge technologies and their real-world applications.
The evolution of artificial heart technology has been driven by the persistent need to address the global shortage of donor hearts and the limitations of current ventricular assist devices. The BiVacor TAH aims to overcome these challenges by offering a fully implantable, durable, and potentially permanent solution. Its development is rooted in the convergence of advanced materials science, miniaturized electronics, and sophisticated fluid dynamics modeling.
The primary objectives of the BiVacor TAH research are multifaceted. Firstly, it seeks to create a device that can provide long-term circulatory support with minimal complications, such as thrombosis, infection, and device failure. Secondly, the research aims to develop a system that can adapt to varying physiological demands, mimicking the natural heart’s ability to respond to changes in activity levels and metabolic needs. Thirdly, the BiVacor TAH project strives to minimize the device’s size and weight, making it suitable for a broader range of patients, including smaller adults and potentially pediatric cases.
Another critical goal of the BiVacor TAH research is to improve the quality of life for recipients. This includes reducing the need for external power sources, minimizing noise and vibration, and enabling patients to engage in a wider range of daily activities. The technology also aims to incorporate advanced monitoring and control systems, allowing for remote management and early detection of potential issues.
From a technical perspective, the BiVacor TAH research focuses on developing a magnetically levitated, continuous-flow pump system that can provide pulsatile flow when needed. This approach aims to combine the durability advantages of continuous-flow devices with the physiological benefits of pulsatile circulation. The research also encompasses the development of biocompatible materials and surface treatments to reduce the risk of blood clots and improve overall hemocompatibility.

As the field of artificial hearts continues to evolve, the BiVacor TAH research represents a significant step towards creating a truly viable alternative to heart transplantation. By addressing the limitations of previous artificial heart designs and incorporating cutting-edge technologies, this research aims to set new standards in the treatment of end-stage heart failure and potentially revolutionize the field of cardiac care.
Market Need Analysis for Artificial Hearts
The global market for artificial hearts has been experiencing significant growth, driven by the increasing prevalence of cardiovascular diseases and the shortage of donor hearts for transplantation. The BiVacor Total Artificial Heart (TAH) represents a promising solution to address this critical need. Market analysis indicates a substantial demand for advanced artificial heart technologies, with the potential to revolutionize the treatment of end-stage heart failure.
The artificial heart market is projected to expand at a compound annual growth rate (CAGR) of over 10% in the coming years, reflecting the urgent need for innovative cardiac support devices. Factors contributing to this growth include the rising incidence of heart failure, an aging population, and advancements in medical technology. The BiVacor TAH, with its unique centrifugal pump design and magnetically levitated rotor, is well-positioned to capture a significant share of this expanding market.
Current estimates suggest that approximately 26 million people worldwide suffer from heart failure, with a subset of these patients requiring advanced therapies such as heart transplantation or mechanical circulatory support. However, the limited availability of donor hearts creates a substantial gap between supply and demand. In the United States alone, it is estimated that only about 3,500 heart transplants are performed annually, while the number of patients on the waiting list far exceeds this figure.
The BiVacor TAH addresses several key market needs that are not fully met by existing solutions. Its compact size and potential for long-term use make it suitable for a wider range of patients, including those with smaller body sizes who may not be candidates for other artificial heart devices. Additionally, the device’s ability to adjust blood flow based on the patient’s activity level offers a more physiological approach to cardiac support, potentially improving quality of life for recipients.
Industry trends indicate a growing interest in miniaturization, durability, and biocompatibility of artificial heart devices. The BiVacor TAH aligns well with these trends, offering a smaller form factor and the potential for reduced complications compared to traditional pulsatile artificial hearts. Furthermore, the increasing focus on home-based care and remote monitoring in healthcare creates opportunities for devices like the BiVacor TAH, which could potentially allow for greater patient mobility and reduced hospital stays.
As healthcare systems worldwide grapple with the economic burden of heart failure management, cost-effective solutions that can reduce hospitalization rates and improve patient outcomes are in high demand. The BiVacor TAH’s potential for extended use and lower maintenance requirements could translate into significant cost savings for healthcare providers and payers, further driving market adoption.
Current Challenges in Artificial Heart Technology
Artificial heart technology has made significant strides in recent years, yet several challenges persist in the development and implementation of total artificial hearts (TAHs) like the BiVacor. One of the primary challenges is achieving long-term durability and reliability. Current TAHs are designed to function for extended periods, but issues such as mechanical wear, material degradation, and the potential for device failure remain significant concerns. Engineers and researchers are continuously working to improve the longevity of these devices, exploring advanced materials and innovative designs to enhance their durability.
Another critical challenge is the management of blood flow and prevention of thrombosis. The artificial heart must maintain proper blood flow dynamics to prevent clot formation, which can lead to severe complications. Achieving the right balance between sufficient flow and minimal blood damage is a complex task that requires sophisticated fluid dynamics modeling and extensive testing. Additionally, the development of biocompatible surfaces that resist clot formation while maintaining optimal blood flow characteristics remains an ongoing area of research.
Power supply and energy efficiency present another set of challenges. Current TAHs rely on external power sources, which limit patient mobility and quality of life. The development of more efficient and compact power systems, including improved batteries and transcutaneous energy transfer systems, is crucial for enhancing patient independence and reducing the risk of infection associated with driveline exits.
Size and fit remain significant hurdles in artificial heart technology. The BiVacor and other TAHs must be designed to accommodate a wide range of patient sizes and anatomies. Creating a device that is small enough to fit in most patients while still providing adequate cardiac output is a delicate balance. This challenge is particularly acute in pediatric patients and smaller adults, where space constraints are more pronounced.
Biocompatibility and immune response management continue to be areas of concern. Despite advances in materials science, the long-term presence of artificial materials in the body can trigger immune responses and inflammation. Researchers are exploring new biomaterials and surface treatments to mitigate these issues and improve the overall biocompatibility of TAHs.

Control systems and physiological responsiveness represent another frontier in artificial heart technology. Developing algorithms and sensors that can accurately mimic the natural heart’s response to varying physiological demands, such as during exercise or stress, remains a complex challenge. The ability to seamlessly adjust cardiac output based on the body’s needs is crucial for providing patients with a near-normal quality of life.
Lastly, the regulatory pathway and clinical validation process for TAHs are rigorous and time-consuming. Demonstrating long-term safety and efficacy in human trials is essential but challenging, given the critical nature of the device and the ethical considerations involved in testing such life-sustaining technology.
Artificial Heart Development Timeline
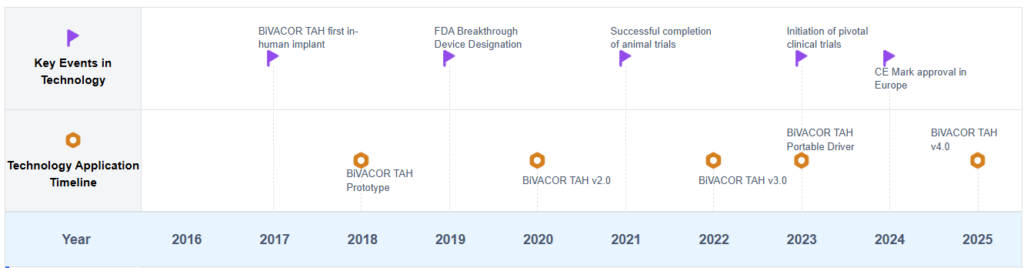
Key Players in Artificial Heart Industry
The research on BiVacor Total Artificial Heart is in an early development stage, with a growing market potential as the demand for heart transplant alternatives increases. The technology is still evolving, with various companies and institutions contributing to its advancement. Key players like The Cleveland Clinic Foundation, SynCardia Systems LLC, and Berlin Heart GmbH are at the forefront of this research. While the technology shows promise, it is not yet fully mature, with ongoing clinical trials and regulatory approvals pending. The competitive landscape is characterized by a mix of established medical device companies and innovative startups, each working to overcome the challenges of creating a reliable, long-term artificial heart solution.
 SynCardia Systems LLC
SynCardia Systems LLC
Technical Solution
SynCardia Systems LLC has developed the SynCardia temporary Total Artificial Heart (TAH), which is currently the only FDA-approved TAH. Their system replaces both failing heart ventricles and four heart valves, providing immediate, safe blood flow of up to 9.5 liters per minute through both ventricles. The device is designed to eliminate the symptoms and source of end-stage biventricular heart failure, bridging patients to heart transplants. SynCardia’s TAH uses pneumatic drivers to power its pumping action, with external batteries and controllers allowing for patient mobility.
Strengths: FDA-approved, proven clinical efficacy, immediate restoration of blood flow.
Weaknesses: External power source limits patient mobility, potential for infection at driveline site, not a permanent solution.
 Berlin Heart GmbH
Berlin Heart GmbH
Technical Solution
Berlin Heart GmbH has developed the EXCOR Adult, a ventricular assist device (VAD) system that can be used as a bridge to transplantation or recovery. While not a total artificial heart, their technology is relevant to the field of mechanical circulatory support. The EXCOR Adult system consists of one or two extracorporeal blood pumps, cannulae for connection to the heart and blood vessels, and a driving unit. The system can be used for left, right, or biventricular support, making it versatile for different patient needs. Berlin Heart’s pneumatic drive systems allow for pulsatile blood flow, mimicking the natural heart’s action.
Strengths: Versatile support options, pulsatile flow mimics natural heart, available in various sizes. Weaknesses: Not a total artificial heart, external components may limit patient mobility, requires anticoagulation therapy.
 Chengdu Sailanuo Medtech Co., Ltd.
Chengdu Sailanuo Medtech Co., Ltd.
Technical Solution
Chengdu Sailanuo Medtech Co., Ltd. is developing an innovative Total Artificial Heart system that aims to address some of the limitations of current TAH designs. Their approach focuses on creating a more compact and efficient device that can be fully implanted. The company’s TAH design incorporates advanced fluid dynamics to optimize blood flow and reduce the risk of thrombosis. They are also working on integrating smart control systems that can adapt the device’s output to the patient’s physiological needs. Sailanuo’s TAH aims to provide long-term support with improved biocompatibility and reduced risk of complications.
Strengths: Compact design for full implantation, smart control systems for adaptive output.
Weaknesses: Limited clinical data available, challenges in long-term power supply and durability.
 Scandinavian Real Heart AB
Scandinavian Real Heart AB
Technical Solution
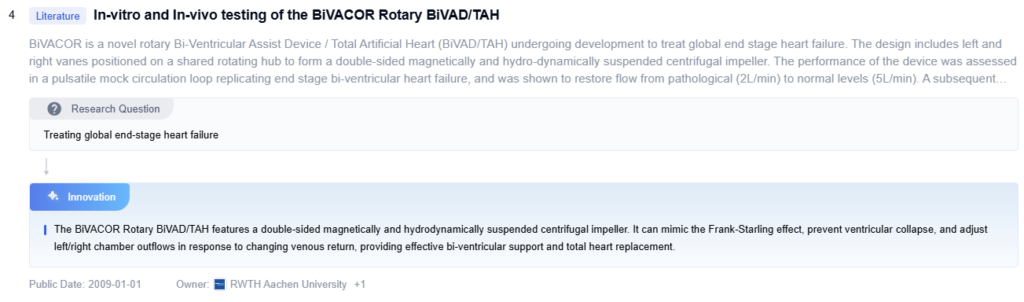
Scandinavian Real Heart AB is developing a Total Artificial Heart that aims to mimic the natural heart’s function more closely than existing devices. Their TAH design features four chambers, similar to the human heart, which they believe will provide more physiological blood flow patterns. The device uses an atrio-ventricular plane displacement principle, which is intended to reduce energy consumption and minimize blood trauma. Scandinavian Real Heart’s TAH incorporates sensors to monitor and adjust its function based on the patient’s activity level and physiological needs. The company is focusing on creating a fully implantable system with improved hemocompatibility and long-term durability.
Strengths: Four-chamber design mimics natural heart function, energy-efficient operation.
Weaknesses: Still in development phase, challenges in miniaturization and long-term reliability.
BiVacor TAH Technical Specifications
Design and structure of BiVacor Total Artificial Heart
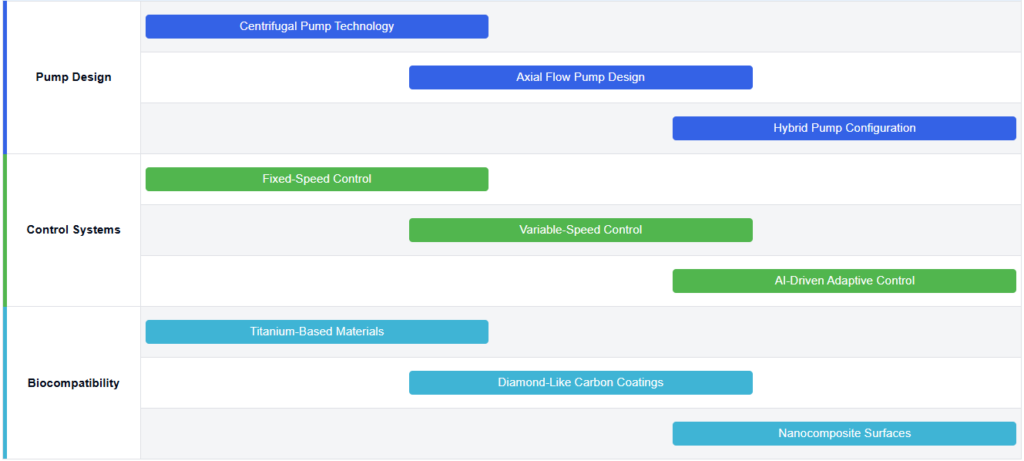
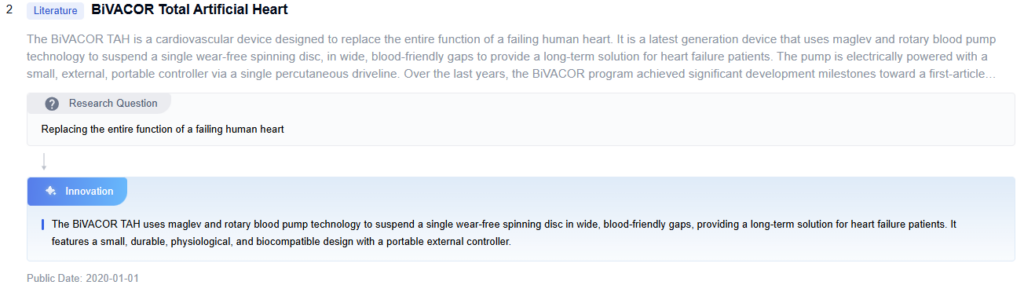
- The BiVacor Total Artificial Heart is a novel device designed to replace the function of a failing heart. It features a compact, single-piece design with a magnetically levitated rotor that can provide both left and right heart support. The device aims to improve upon traditional artificial hearts by offering a more durable and efficient solution for patients with end-stage heart failure.
- Centrifugal pump design for BiVacor Total Artificial Heart
The BiVacor Total Artificial Heart utilizes a centrifugal pump design to provide continuous blood flow. This design incorporates a magnetically levitated impeller that can be precisely controlled to adjust flow rates and pressure. The centrifugal pump allows for a compact and efficient artificial heart structure, capable of supporting both left and right ventricular functions. - Magnetic levitation system in BiVacor Total Artificial Heart
A key feature of the BiVacor Total Artificial Heart is its magnetic levitation system. This system suspends the impeller within the pump housing, eliminating the need for mechanical bearings. The magnetic levitation reduces friction, minimizes blood damage, and enhances the device’s durability. It allows for precise control of the impeller’s position and rotation, optimizing blood flow dynamics. - Control and monitoring systems for BiVacor Total Artificial Heart
The BiVacor Total Artificial Heart incorporates advanced control and monitoring systems. These systems continuously adjust the pump’s performance based on the patient’s physiological needs. They monitor parameters such as blood flow, pressure, and power consumption, and can adapt to changes in the patient’s activity level or position. The control systems also include safety features to detect and respond to potential malfunctions. - Implantation and integration of BiVacor Total Artificial Heart
The design of the BiVacor Total Artificial Heart focuses on ease of implantation and integration with the patient’s cardiovascular system. It features connectors and interfaces that allow for secure attachment to the major blood vessels. The device’s compact size and shape are optimized to fit within the chest cavity, replacing the native heart. Consideration is given to the placement of power and control lines to exit the body with minimal risk of infection.
Control systems and algorithms for artificial hearts
Advanced control systems and algorithms are crucial for the optimal functioning of artificial hearts, including the BiVacor device. These systems regulate blood flow, adjust pump speed, and monitor various physiological parameters to ensure proper circulation and adapt to the patient’s changing needs. Machine learning and AI technologies are being incorporated to enhance the device’s responsiveness and efficiency.
Power supply and energy management
Efficient power supply and energy management are critical aspects of artificial heart technology. Innovations in this area focus on developing long-lasting, rechargeable batteries, wireless power transmission systems, and energy-efficient components to extend the device’s operating time and improve patient mobility and quality of life.
Biocompatibility and materials science
The development of biocompatible materials is essential for the success of artificial heart devices. Research in this area focuses on creating materials that minimize the risk of blood clotting, reduce inflammation, and improve the overall integration of the device with the patient’s body. Advanced coatings and surface treatments are being explored to enhance the long-term performance and safety of artificial hearts.
Implantation techniques and surgical procedures
Advancements in implantation techniques and surgical procedures are crucial for the successful deployment of artificial hearts like the BiVacor device. These innovations aim to minimize invasiveness, reduce recovery time, and improve overall patient outcomes. New approaches include minimally invasive surgical techniques, improved anastomosis methods, and optimized post-operative care protocols.
Core Innovations in BiVacor TAH Design


Future Directions in Artificial Heart Research
Advanced Biocompatible Materials
Advanced biocompatible materials are transforming the future of the BiVacor Total Artificial Heart (TAH). Researchers now focus on creating materials that integrate naturally with the human body. These innovations help reduce rejection and other complications. By mimicking the properties of natural heart tissue, these materials improve both performance and lifespan.
One promising direction involves using nanocomposite materials. These combine strong synthetic polymers with the biocompatibility of natural substances. Engineers can design them with self-healing properties to extend the device’s lifespan. This could lower the need for future replacement surgeries.
In addition, scientists are developing surface treatments to prevent blood clot formation. These treatments aim to reduce the risk of thromboembolism. Some approaches include embedding heparin-like molecules into the material. Others use micro-textured surfaces to discourage platelet adhesion.
Researchers are also exploring hybrid materials that include living cells. They may seed the artificial heart with a patient’s stem cells. These cells can then grow into cardiac tissue. This strategy could create a more natural connection between the device and the body. As a result, it may improve function and lower immune responses.
The innovation doesn’t stop with the heart’s structure. Scientists are redesigning the BiVacor TAH’s power systems using flexible, biocompatible batteries. These batteries can fit better within the device and improve patient comfort. Some concepts even aim to recharge the batteries using the body’s metabolic processes. This would reduce dependence on external power sources and boost overall autonomy.
Strengths: These advancements offer stronger biocompatibility and lower rejection risks. They also promise longer device life and better body integration. Some materials may even repair themselves over time.
Weaknesses: Despite the potential, challenges remain. Development costs are high, and regulatory approval is complex. Long-term effects are still unknown. Scaling up production of these advanced materials also poses difficulties.
Regulatory Pathway for Artificial Hearts
The regulatory process for artificial hearts, including the BiVacor Total Artificial Heart, is complex and highly structured. This pathway ensures that these life-saving devices are safe and effective for patients. In the United States, the Food and Drug Administration (FDA) oversees this process. Artificial hearts fall under Class III medical devices due to their life-sustaining role and higher risks.
The journey begins with preclinical testing. Manufacturers conduct in vitro and in vivo studies to prove safety and performance. After gathering enough data, they can submit an Investigational Device Exemption (IDE) application to the FDA. This application allows them to begin clinical trials in human patients.
Clinical trials follow a phased approach. First, small-scale feasibility studies assess initial safety. Then, larger pivotal trials measure long-term efficacy and compare the device to current treatments. Throughout each phase, manufacturers must follow strict guidelines and report all device malfunctions or adverse events.
After completing clinical trials, the next step is submitting a Premarket Approval (PMA) application. This document includes all preclinical and clinical results, manufacturing details, and product labeling. The FDA then conducts a thorough review, often with help from outside experts. If the device meets all standards, the FDA grants approval for market release.
Even after approval, monitoring continues. Manufacturers must follow post-market surveillance rules. These include ongoing data collection and immediate reporting of any complications or failures. This ensures continued patient safety.
For devices like the BiVacor Total Artificial Heart, the FDA may provide special support. The Breakthrough Devices Program helps speed up review for technologies that offer better treatment for serious conditions. Because of its unique design, the BiVacor TAH might also require additional testing and data.
Outside the U.S., other countries follow similar paths. Many align with FDA standards or follow the European Medicines Agency (EMA). In Europe, artificial hearts must meet the Medical Device Regulation (MDR). Devices must also earn a CE mark before entering the market. Approval involves clinical studies and technical reviews by Notified Bodies.
As technology advances, regulatory bodies continue to adapt. They work to balance innovation with patient safety. Because of this, manufacturers like BiVacor must stay in close contact with regulators. This helps ensure compliance and a smooth path to market approval.
Biocompatibility and Long-Term Durability
Biocompatibility and durability are critical to the success of the BiVacor Total Artificial Heart (TAH). As a fully implantable heart device, it must work in harmony with the human body and function reliably for years.
Biocompatibility remains one of the top priorities during development. The BiVacor TAH must not trigger immune responses or cause harmful side effects. To avoid complications like inflammation or blood clots, engineers use proven materials. Titanium alloys and medical-grade polymers offer excellent compatibility and strength.
However, researchers continue to enhance these materials. They develop advanced coatings and surface treatments to reduce infection risks. These modifications also help the device integrate better with surrounding tissues.
Equally important is long-term durability. The BiVacor TAH must run nonstop for years without failure. To support this, the device uses a magnetically levitated impeller. This design minimizes friction and wear, which may extend the system’s lifespan.
Researchers run extensive lab tests and animal trials to prove durability. They study how the device handles stress, corrosion, and magnetic stability over time. These tests simulate real-world conditions inside the body.
One big challenge is the effect of constant blood flow. The TAH must resist damage while staying free of clots and blockages. To tackle this, engineers test anti-thrombogenic coatings and surface modifications. These enhancements aim to reduce clot risk and improve performance.
Durability also depends on reliable power and control systems. The BiVacor TAH uses external power and smart algorithms to manage blood flow. Making these systems more efficient is a top research goal. Teams work on better power delivery and tougher control software.
As the TAH moves through clinical testing, long-term human trials will become essential. These studies will track how well the device performs over time. The data will guide future upgrades and help reduce risks.
Ultimately, the goal is clear. BiVacor aims to deliver a heart that lasts, supports life continuously, and minimizes complications. For patients with end-stage heart failure, this technology could provide a dependable alternative to transplantation.
To get detailed scientific explanations of BiVacor Total Artificial Heart, try Patsnap Eureka.


