Overview
Levothyroxine Sodium, marketed as Euthyrox in many countries, is one of the most widely prescribed medications in the US market. The US market has 1 approved drug for Levothyroxine Sodium, with numerous formulations, strengths, and manufacturers. This medication is primarily used for the treatment of hypothyroidism, hyperpituitarism, myxedema coma, and other thyroid-related conditions. The drug has been available for decades and has a mature market with multiple generic versions available. Despite being a well-established drug, it maintains a significant market presence due to the chronic nature of hypothyroidism requiring lifelong treatment.
Detailed Description
Drug Information
Levothyroxine Sodium was originally developed by AbbVie, Inc. and has been approved in the USA in various formulations:

The drug is also approved and marketed by several other companies including Jerome Stevens Pharmaceuticals, Inc., Cediprof, Inc., King Pharmaceuticals, EMD Serono, Inc., and Genus Lifesciences, Inc., among others.
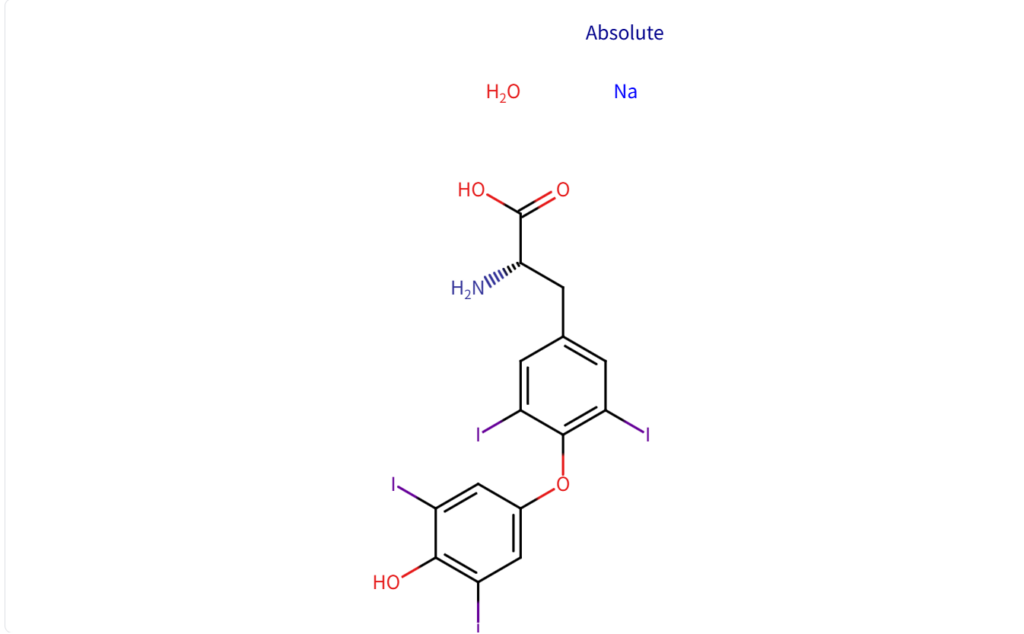
Structure
Patent Statement/Paragraph IV
There are multiple patent statements for Levothyroxine Sodium products in the US:
For Levoxyl (King Pharmaceuticals):
| Product Name | Submission Date | Number of ANDAs |
|---|---|---|
| Levoxyl | Pre-MMA | Not specified |
For Tirosint (IBSA Institut Biochimique SA):

For Thyquidity (Jerome Stevens Pharmaceuticals):
| Product Name | Submission Date | Number of ANDAs | 180-day exclusivity status | First Applicant Approval Date |
|---|---|---|---|---|
| Thyquidity | 12/28/2022 | 1 | Extinguished | 6/9/2025 |
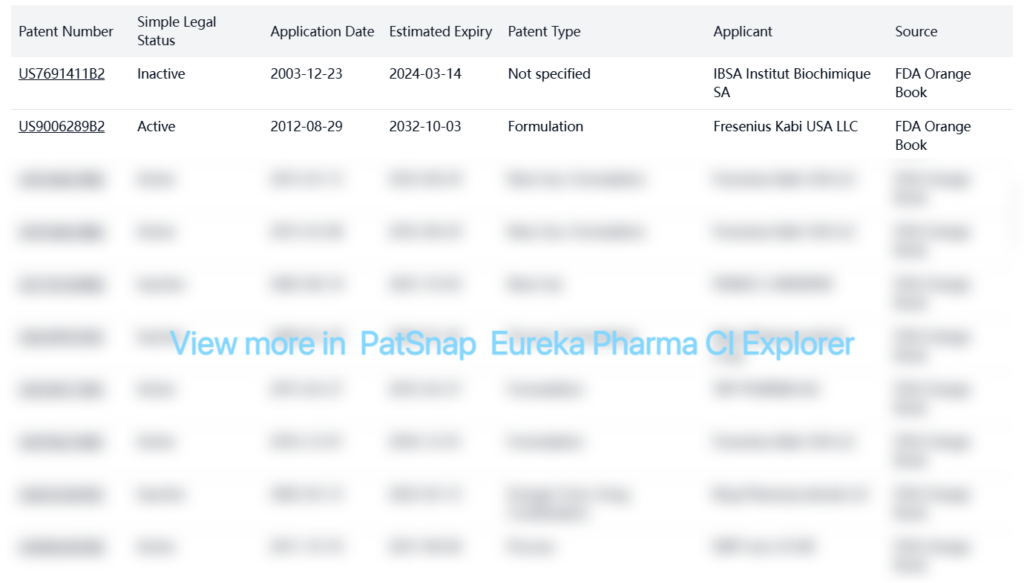
Registration Patent Barrier Analysis
FDA Orange Book patents for Levothyroxine Sodium include:
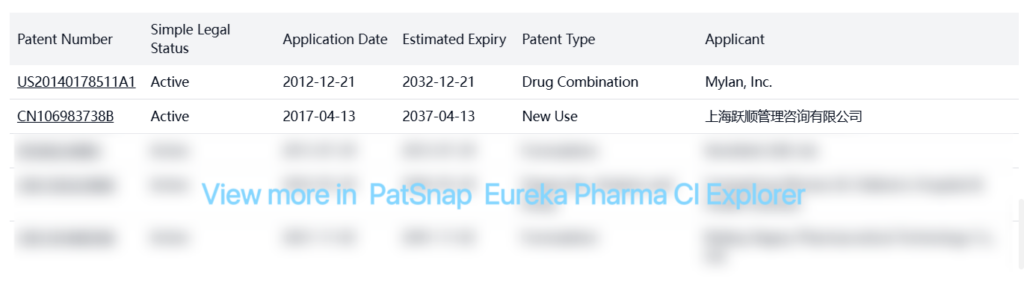
Other Patent Barrier Analysis
There are numerous other patents related to Levothyroxine Sodium that are not listed in the FDA Orange Book. These include patents covering formulation improvements, manufacturing processes, and new uses. Some notable patents include:
Clinical Results
Based on the FDA Label Clinical Insight, levothyroxine sodium has been extensively studied in various clinical settings:
- Pharmacodynamics and Mechanism of Action:
- Mechanistic studies have demonstrated that levothyroxine is converted to the physiologically active thyroid hormone (triiodothyronine, T3) in tissues.
- This conversion and binding to nuclear thyroid receptors underpin its effects on metabolism and gene expression.
- Pharmacokinetics:
- Detailed experiments have characterized the pharmacokinetic profile of levothyroxine sodium.
- Studies have measured absorption rates, peak plasma concentrations, and half-life in both healthy subjects and patients with thyroid dysfunction.
- Population-based pharmacokinetic studies have been conducted in pediatric, adult, and geriatric patients to understand variations in metabolism and clearance.
- Nonclinical Toxicology:
- Long-term animal studies have assessed potential carcinogenicity and mutagenicity.
- Reproductive toxicity studies have evaluated its impact on fertility.
- Controlled overdosage experiments have established safety boundaries.
- Drug Interaction Experiments:
- Studies have explored interactions with oral anticoagulants, digitalis glycosides, and sympathomimetic amines.
- Additional research has examined interactions in special populations and with concomitant medications.
Infringement Cases
Based on the search results, there are no reported patent infringement incidents involving levothyroxine sodium. The available references focus on regulatory guidance for conducting pharmacokinetic, bioavailability, and bioequivalence studies as well as the submission of applications for levothyroxine sodium products.
Policy and Regulatory Risk Warning
After a comprehensive search, Levothyroxine Sodium does not appear to have any current market exclusivity or data protection period in the United States. Most of the original patents covering the compound have expired, allowing for generic competition. However, specific formulations and delivery methods are still protected by patents that will expire between 2024 and 2039.
The FDA has established narrow bioequivalence criteria for levothyroxine products due to its narrow therapeutic index. Generic manufacturers must demonstrate that their products meet these strict criteria, which can pose a regulatory hurdle.
Market Entry Assessment & Recommendations
Based on the comprehensive analysis of Levothyroxine Sodium in the US market, here are the key assessments and recommendations:
- Patent Landscape Assessment:
- The basic compound patents for Levothyroxine Sodium have expired, allowing generic entry.
- However, numerous formulation patents remain active until 2039, particularly for specialized delivery forms (liquids, capsules, injectables).
- Companies should focus on developing formulations that don’t infringe on existing patents or consider challenging specific patents if appropriate.
- Market Differentiation Strategies:
- Develop improved formulations with better stability or bioavailability profiles.
- Consider alternative delivery systems that might provide patient benefits (e.g., orally disintegrating tablets for patients with swallowing difficulties).
- Invest in patient education and support programs to build brand loyalty in this chronic medication market.
- Regulatory Strategy:
- Be prepared for stringent bioequivalence requirements due to the narrow therapeutic index of Levothyroxine.
- Consider conducting additional clinical studies to support marketing claims about improved absorption or reduced variability.
- Engage with the FDA early in the development process to align on expectations for approval.
- Market Entry Timing:
- For generic tablet formulations, immediate market entry is possible as basic patents have expired.
- For specialized formulations (like Tirosint capsules or liquid formulations), plan entry timing based on specific patent expiries.
- Monitor ongoing patent litigation that might affect the patent landscape.
- Pricing and Reimbursement Strategy:
- Despite being a generic market, maintain competitive pricing while emphasizing quality and consistency of the product.
- Develop relationships with pharmacy benefit managers and insurers to secure favorable formulary placement.
- Consider patient assistance programs to improve access and build brand recognition.
- Manufacturing Considerations:
- Ensure robust manufacturing processes that can consistently produce product meeting tight specification limits.
- Consider implementing additional quality controls beyond regulatory requirements to differentiate on quality.
- Invest in supply chain security to avoid stockouts in this essential medication category.
- Post-Marketing Surveillance:
- Implement comprehensive pharmacovigilance programs to monitor adverse events.
- Collect real-world evidence on product performance to support marketing claims.
- Engage with endocrinologists and other prescribers to gather feedback on product performance.
The Levothyroxine Sodium market, while mature and competitive, still offers opportunities for companies that can differentiate their products through quality, formulation innovation, or patient-centric approaches. Strategic patent navigation and regulatory expertise will be key success factors for new market entrants.
For more scientific and detailed information of Euthyrox (Levothyroxine Sodium), try PatSnap Eureka Pharma CI Explorer.