
Famotidine, a second-generation H2-receptor antagonist, has been a mainstay in the treatment of acid-related gastrointestinal conditions for decades. With strong clinical efficacy, rapid onset of action, and excellent safety, famotidine continues to be widely used across both prescription and over-the-counter (OTC) segments for GERD, peptic ulcers, and gastric hypersecretion syndromes.
This article offers a comprehensive review of famotidine’s pharmacological profile, clinical applications, and evolving role in GI therapy—while also demonstrating how the PatSnap Eureka AI Agent empowers researchers and healthcare professionals to uncover deeper insights into molecules like famotidine.
What is Famotidine?
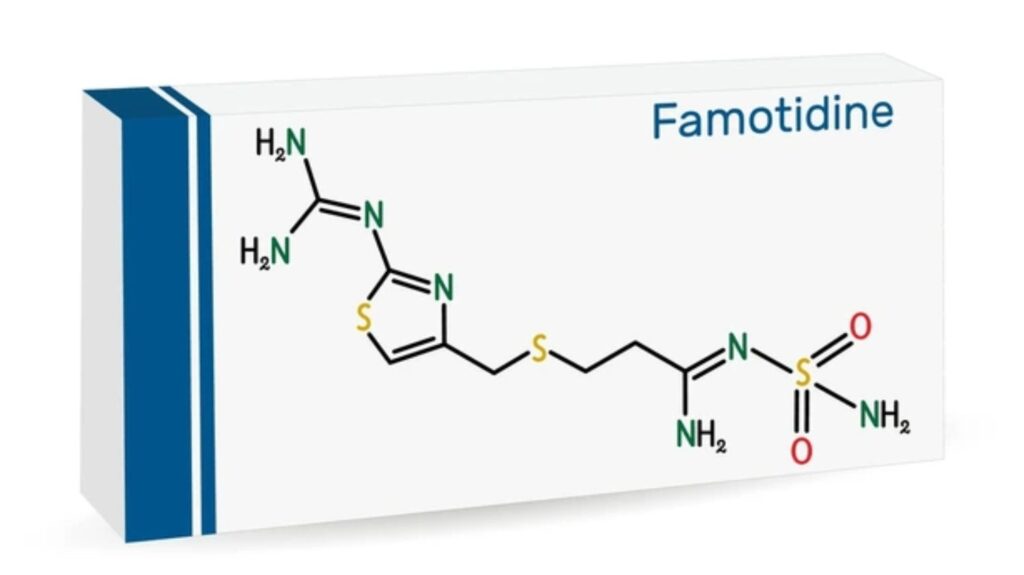
Introduced in the 1980s and marketed under brands such as Pepcid, famotidine has been widely prescribed for both short-term relief and long-term acid control. Famotidine works by blocking histamine from stimulating the parietal cells in the stomach lining, thus lowering gastric acid secretion. It is a medication that belongs to a class of drugs known as H2 receptor antagonists (or H2 blockers). It is primarily used to treat conditions related to excess stomach acid, such as:
Zollinger-Ellison Syndrome: This is a rare condition where a tumor in the pancreas or duodenum causes excessive gastric acid production. Famotidine helps manage the high levels of acid.
Gastroesophageal Reflux Disease (GERD): Famotidine helps reduce the amount of acid produced in the stomach, which can alleviate symptoms like heartburn and acid indigestion. It is available over-the-counter (OTC) for this purpose, often under brand names like Pepcid AC.
Peptic Ulcers: Famotidine can help heal ulcers in the stomach (gastric ulcers) and the duodenum (duodenal ulcers) by reducing acid levels, allowing the lining of the digestive tract to heal. It is also used to prevent the recurrence of duodenal ulcers.
Key Characteristics of Famotidine
- Drug Class: H2 (histamine-2) receptor antagonist
- Mechanism: Inhibits gastric acid secretion by blocking H2 receptors on parietal cells
- Common Brand Name: Pepcid
- FDA Status: Approved since 1986
- OTC and Prescription: Available in both forms
- COVID-19 Exploration: Studied for anti-inflammatory and antiviral properties (off-label use)
Key Forms and Formulations
| Form | Strengths | Route | Notes |
|---|---|---|---|
| Tablet (OTC) | 10 mg, 20 mg | Oral | For heartburn and indigestion |
| Tablet (Rx) | 20 mg, 40 mg | Oral | GERD and ulcer treatment |
| Oral Suspension | 40 mg/5 mL | Oral | Pediatric or dysphagia use |
| Injectable form | 20 mg/2 mL | IV | For acute hospital-based treatment |
Mechanism of Action
Famotidine is N’-(aminosulfonyl)-3-[[[2-[(diaminomethylene)amino]-4-thiazolyl]methyl]thio] propanimidamide. It works by restricting the measure of destructive conveys by stomach. Famotidine is used to treat and prevent stomach ulcer and absorption tracts ulcer. It furthermore used to treat conditions in which the stomach makes too much destructive, for instance, Zollinger-Ellison issue. Famotidine also treats gastroesophageal reflux disease (GERD) and diverse conditions in which destructive goes down from the stomach into the throat, causing acid reflux.
Detailed Mechanism
Histamine H2-Receptor Antagonism
Famotidine acts by binding directly to histamine H2-receptors on the basolateral membrane of gastric parietal cells. Under normal conditions, histamine—released from enterochromaffin-like (ECL) cells in response to triggers like food, caffeine, insulin, or pentagastrin—activates these receptors. This activation turns on adenylate cyclase, which raises intracellular cAMP levels. Higher cAMP levels activate protein kinase A, which then stimulates the H⁺/K⁺-ATPase proton pump on the apical membrane. This final step drives gastric acid secretion into the stomach lumen.
Inhibition of Gastric Acid Secretion
Famotidine blocks this cascade early. By occupying H2-receptors, it prevents histamine from binding. As a result, adenylate cyclase remains inactive, cAMP levels stay low, and protein kinase A is not activated. This chain reaction inhibits the proton pump, cutting acid secretion at its source. Famotidine reduces both basal and nocturnal acid output. It also dampens acid secretion triggered by food, caffeine, insulin, and pentagastrin. In addition, it lowers gastric volume and acidity, improving symptoms and protecting mucosal surfaces.
Reduction of Pepsin Secretion
Famotidine also reduces pepsin output, an enzyme essential for protein digestion in the stomach. Since pepsin secretion depends on acid volume, its reduction correlates directly with the drop in gastric secretion. This dual action—acid and pepsin control—enhances its protective role in acid-related disorders.
- Anti-Inflammatory Effects: Famotidine exerts anti-inflammatory effects through a vagus nerve-dependent mechanism. It activates the inflammatory reflex—a brain-integrated neural circuit that suppresses inflammation via α7 nicotinic acetylcholine receptor (α7nAChR) signaling. When administered intraperitoneally, famotidine significantly reduced serum and splenic levels of LPS-stimulated tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6). This reduction improved survival rates in animal models. Central administration via intracerebroventricular (ICV) injection produced even stronger anti-inflammatory effects, highlighting a central nervous system component to its action. Famotidine’s efficacy did not depend on mast cells. Mice genetically engineered to lack mast cells still showed a strong anti-inflammatory response, suggesting a mast cell-independent pathway. However, when researchers performed bilateral sub-diaphragmatic vagotomy or used α7nAChR knockout mice, famotidine lost its anti-inflammatory activity. This clearly confirms that the inflammatory reflex, mediated via vagal and α7nAChR pathways, is central to famotidine’s mechanism of action in inflammation control.
Therapeutic Implications
The inhibition of gastric acid secretion by famotidine makes it effective in the treatment of various acid-related gastrointestinal disorders. These include:
- Gastroesophageal Reflux Disease (GERD): Famotidine is used to treat symptoms such as heartburn and acid indigestion. It is available over-the-counter (OTC) for the prevention and relief of heartburn associated with acid indigestion and sour stomach.
- Peptic Ulcers: Famotidine is used to treat active duodenal and gastric ulcers. It can also be used to reduce the risk of duodenal ulcer recurrence.
- Zollinger-Ellison Syndrome: Famotidine is used to treat pathological hypersecretory conditions, such as Zollinger-Ellison Syndrome, where there is excessive gastric acid production.
- Prevention of NSAID-Induced Ulcers: Famotidine is used in combination with nonsteroidal anti-inflammatory drugs (NSAIDs) to reduce the risk of gastrointestinal ulcers. NSAIDs can cause gastritis, dyspepsia, and gastric and duodenal ulceration due to the inhibition of cyclooxygenase (COX-1 and COX-2) and prostaglandin synthesis. Famotidine helps to protect the stomach lining by reducing acid secretion.
Pharmacokinetics
- Absorption: Famotidine is incompletely absorbed after oral administration, due to its low water solubility and poor lipophilicity. The bioavailability of famotidine from the tablet formulation is only approximately 43%, and is unaffected by food. After oral administration of famotidine, dose-related peak plasma concentration is achieved within 1 to 3.5 hours.
- Distribution: It is distributed widely in the body, with high concentrations found in the digestive tract, kidneys, liver, submandibular glands, and pancreas. It can also be found in breast milk, but it does not readily cross the placental barrier.
- Metabolism: It is metabolized in the body, with the main metabolites being the sulfoxide and sulfone derivatives.
- Excretion: Famotidine is primarily excreted in the urine, with approximately 35-44% of the oral dose and 88-91% of the intravenous dose being excreted unchanged. The half-life of famotidine is approximately 3 hours.
Safety and Tolerance:
Famotidine is generally well tolerated. Most patients experience few adverse reactions. The most common side effects include headache, dizziness, constipation, and diarrhea. This antiulcer drug has a strong safety record. However, rare cases of immunoglobulin E (IgE)-mediated anaphylaxis have been reported. Some patients also show cross-reactivity with nizatidine and ranitidine, likely due to structural similarity. In addition to IgE-mediated reactions, famotidine may trigger anaphylaxis through nonallergic mechanisms.
It may cause direct mediator release from mast cells or basophils, bypassing traditional allergic pathways. Although allergic responses to famotidine have been documented, the underlying mechanisms remain unclear, and no scientific consensus has been reached. Famotidine has not shown systemic effects on the central nervous system, cardiovascular system, respiratory system, or endocrine system in clinical pharmacology studies. It also does not exhibit antiandrogenic activity. In hormone studies, famotidine did not alter serum levels of prolactin, cortisol, thyroxine (T4), or testosterone, further supporting its hormonal safety.
Dosage and Administration
| Indication | Typical Adult Dose |
|---|---|
| GERD | 20 mg twice daily or 40 mg at bedtime |
| Duodenal Ulcer (active) | 40 mg at bedtime for 4–8 weeks |
| Maintenance therapy | 20 mg once daily at bedtime |
| Zollinger–Ellison Syndrome | Starting at 20 mg every 6 hours |
| OTC Use (heartburn relief) | 10–20 mg once or twice daily as needed |
| IV Dosing (hospital use) | 20 mg every 12 hours |
Dosage adjustments are recommended in patients with renal impairment (CrCl < 50 mL/min).
Conclusion
Famotidine remains a foundational therapy for acid-related conditions. With its reliable efficacy, rapid onset, and well-established safety, it continues to serve patients in both acute and chronic care settings. As new roles for famotidine—such as adjunctive therapy in COVID-19 and inflammation-related pathways—emerge, tools like PatSnap Eureka help researchers stay ahead by providing instant access to evolving data, trials, and real-world insights.
In an age of personalized and precision medicine, leveraging structured, AI-powered therapeutic intelligence is essential. Famotidine’s enduring relevance, combined with Eureka’s data clarity, allows professionals to revisit familiar drugs with fresh confidence and clinical precision.
FAQs
No. Both are H2 blockers, but famotidine is more potent and has not been linked to NDMA contamination issues, unlike ranitidine.
This combination is generally not recommended unless under medical supervision, as both suppress acid differently and could increase side effects.
Preliminary studies have explored its anti-inflammatory properties, but it is not approved for COVID-19 treatment.
PatSnap Eureka AI Agent provides access to comparative clinical studies, pharmacodynamic profiles, and regulatory filings to support clinical and research needs.
For a deeper understanding of Famotidine, try PatSnap Eureka AI Agent, which provides detailed intelligence on global clinical trials, pharmacological data, regulatory filings, and market activity related to Famotidine and other H2 receptor antagonists.

