
In chemistry, knowing the maximum amount of product that a reaction can theoretically produce is essential for planning, scaling, and improving lab processes. This maximum output is known as the theoretical yield. Accurately calculating it helps chemists evaluate reaction efficiency, identify losses, and compare actual yield against expectations.
This guide will explain how to calculate theoretical yield in a clear, step-by-step format, while also introducing how PatSnap Eureka can enhance the process with data-driven support.
What Is Theoretical Yield?
What is Surface Plasmon Resonance (SPR)? Eureka Technical Q&A explains that SPR is a powerful optical technique used to detect molecular interactions in real time, widely applied in biosensing, drug discovery, and biochemical research.
Theoretical yield is the amount of product that could be formed from a given amount of reactants under ideal conditions. It assumes complete conversion of the limiting reactant, no side reactions, and 100% efficiency. It is commonly expressed in grams and used as a benchmark to calculate percent yield.
Why Is Theoretical Yield Important?
- It helps researchers optimize material use.
- It provides a target for assessing actual experimental yield.
- It identifies inefficiencies or losses in synthesis.
How to Calculate Theoretical Yield (Step-by-Step with PatSnap Eureka)
Step 1: Write and Balance the Chemical Equation
Start by balancing the chemical equation to ensure the molar ratios between reactants and products are correct.
- With PatSnap Eureka: Eureka gives instant access to validated reaction pathways and balanced equations from scientific databases. This helps verify your equation structure and avoid stoichiometric errors early on.
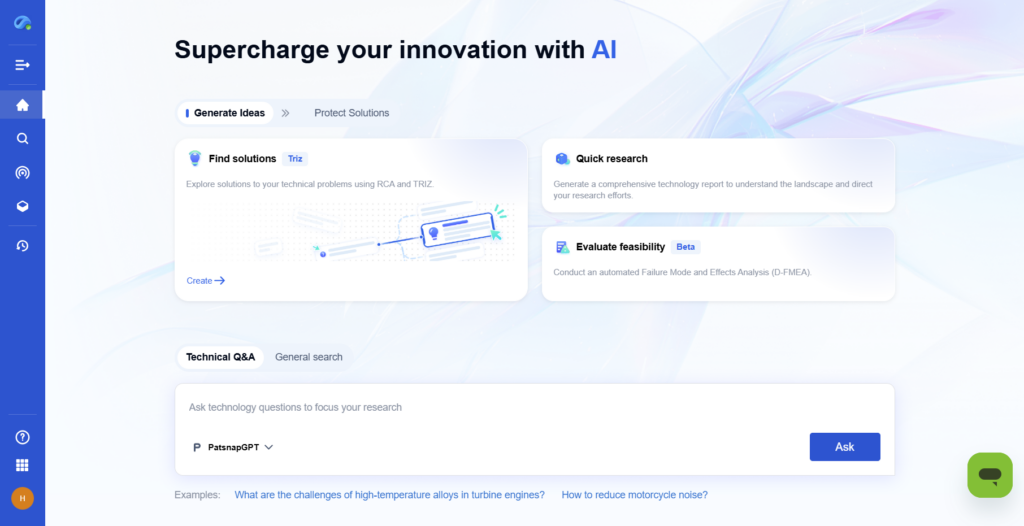
Step 2: Identify the Limiting Reactant
Calculate the moles of each reactant using their molecular weights and compare ratios to determine which one limits the reaction.
- With PatSnap Eureka: Use Eureka’s chemical analysis tools to review previous experimental datasets and spot common limiting reactants in similar reactions. Its AI suggestions assist in identifying constraints.
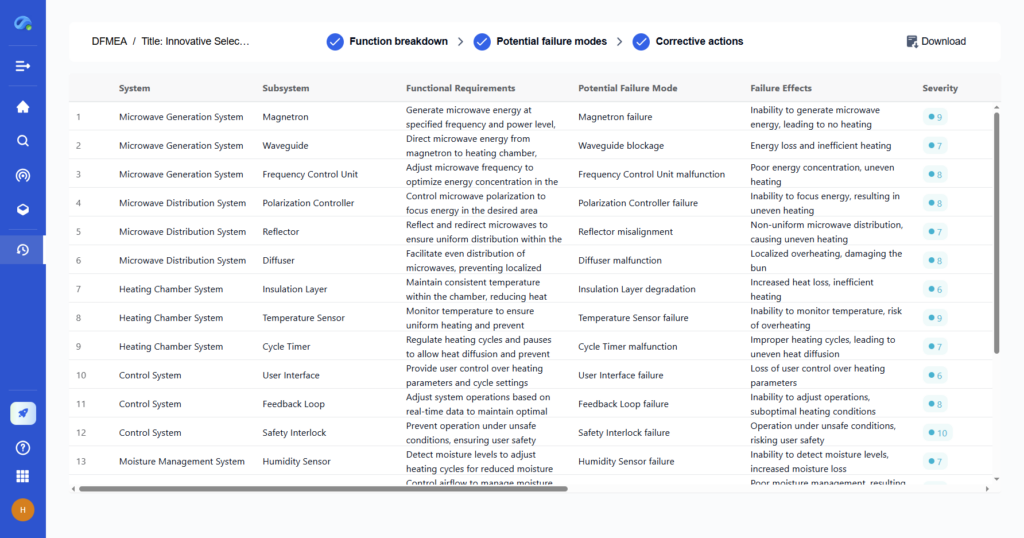
Step 3: Use Stoichiometry to Calculate Product Moles
Apply mole ratios from the balanced equation to convert moles of the limiting reactant into moles of the product.
- With PatSnap Eureka: Eureka can map out stoichiometric relationships across complex, multi-step syntheses using historical lab and patent data to ensure your calculation aligns with empirical results.
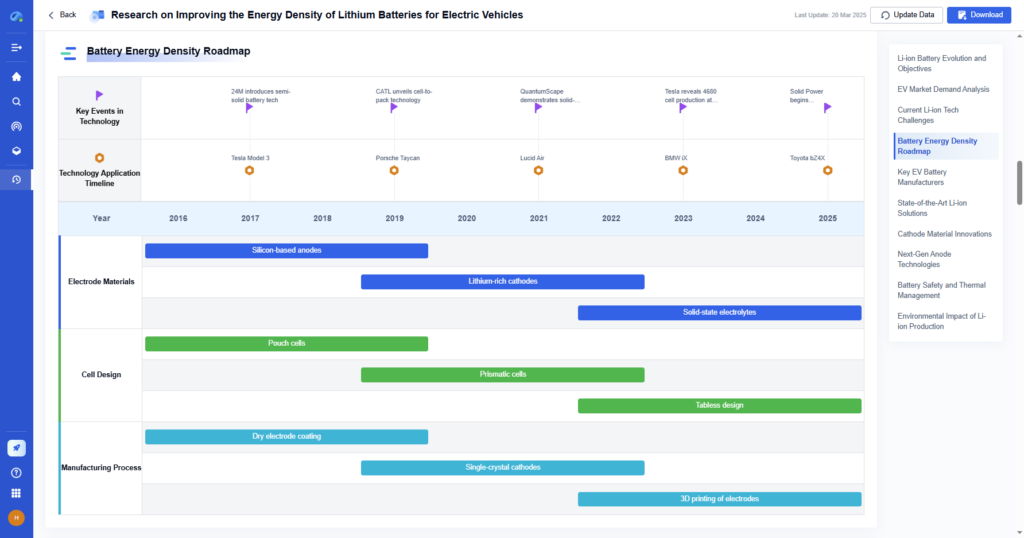
Step 4: Convert Moles to Grams
Multiply the number of product moles by the product’s molar mass to get the theoretical yield in grams.
- With PatSnap Eureka: Eureka provides molar masses and compound-specific data from verified sources, so you can avoid manual lookup errors and maintain precision.
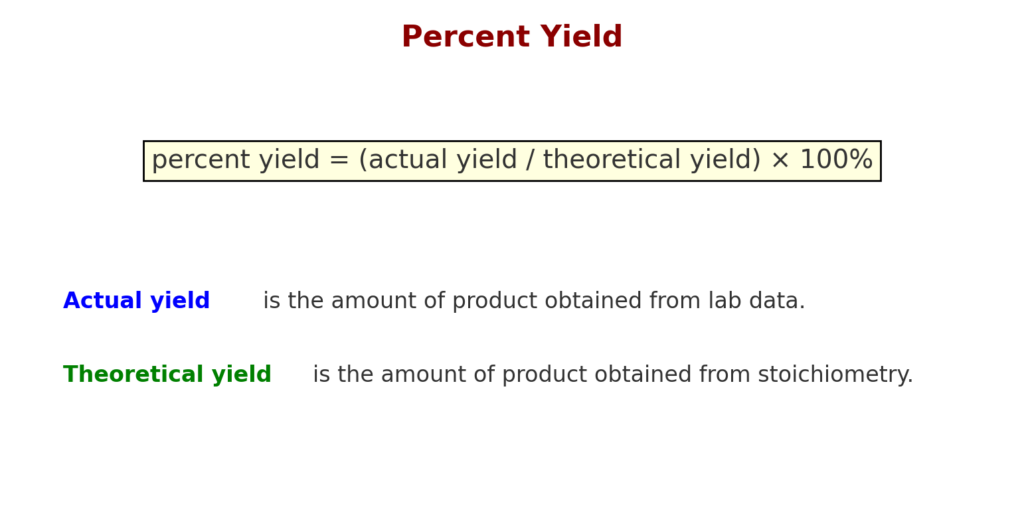
Step 5: Compare with Actual Yield (Optional)
If you’ve already completed the experiment, you can use the theoretical yield to calculate percent yield:
Percent Yield = (Actual Yield / Theoretical Yield) × 100
- With PatSnap Eureka: Track real-time lab outcomes using Eureka’s monitoring tools. Compare historical actual yields across projects and discover potential process improvements.
Example Calculation
Imagine reacting 4 moles of hydrogen (H₂) with 2 moles of oxygen (O₂) to form water (H₂O):
![]()
- Based on the equation, 4 moles of H₂ would produce 4 moles of H₂O.
- The molar mass of water is 18 g/mol.
- Theoretical yield = 4 moles × 18 g/mol = 72 grams of water.
How PatSnap Eureka Improves Theoretical Yield Predictions
PatSnap Eureka goes beyond static calculations:
- Integrated Literature and Patent Data: Access chemical reaction data, yields, and constraints from industry and academic sources.
- Smart Suggestions: AI flags inconsistent or inefficient steps and proposes modifications based on real-world success rates.
- Yield Benchmarks: Compare your estimated yield with published values to determine if your process is realistic or needs adjustment.
- Interactive Reports: Generate visual documentation to support lab reporting, intellectual property filings, or team presentations.
Conclusion
Mastering theoretical yield calculations ensures more efficient experimentation, minimizes material waste, and provides critical insight into reaction performance. With PatSnap Eureka, you gain a competitive edge through data-backed insights, real-time suggestions, and precision at every step of your workflow.
Whether you’re working in academic labs or industrial R&D, combining classic stoichiometry with Eureka’s digital tools will streamline your yield estimation process—and bring smarter results faster.
To get detailed scientific explanations of Theoretical Yield, try Patsnap Eureka.


