
Hydroiodic acid (HI) is a highly corrosive and powerful acid formed by dissolving hydrogen iodide (HI) gas in water. It is known for its strong reducing properties and its role in industrial and laboratory applications, including organic synthesis and iodide production. Due to its strong acidity and reactivity, proper handling and storage are crucial to prevent hazardous exposure. This article explores the properties, uses, hazards, safety measures, and key considerations related to hydroiodic acid.
What is Hydroiodic Acid?
Hydroiodic acid is a hydrogen halide and one of the strongest acids known. It fully dissociates in water, releasing hydrogen ions (H⁺) and iodide ions (I⁻). This complete ionization contributes to its extreme acidity and reactivity, making it useful in chemical and pharmaceutical industries.
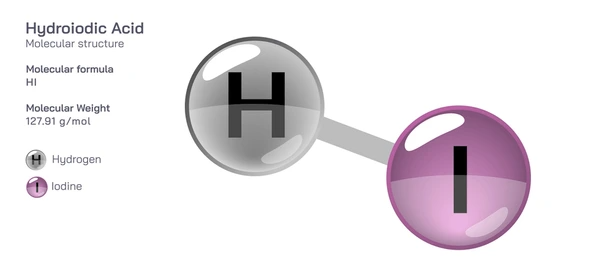
Chemical Properties
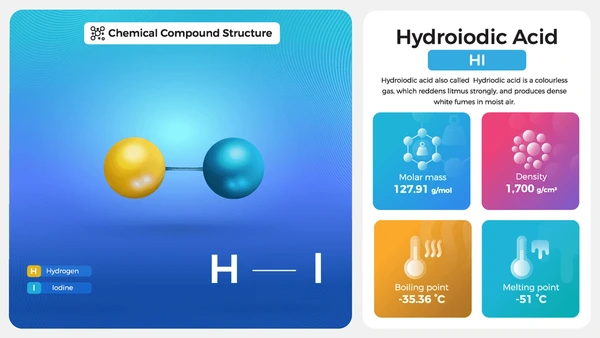
- Molecular Formula: HI
- Molar Mass: 127.91 g/mol
- Appearance: Colorless liquid
- Odor: Pungent, acrid smell
- Density: ~1.70 g/mL (for a 57% solution)
- Boiling Point: ~127°C (57% azeotropic solution)
- Solubility: Highly soluble in water
- Acidity (pKa): ~ -9.3, indicating a very strong acid
Physical States
HI is available in aqueous solutions, with typical concentrations ranging from 48% to 57%. The 57% solution forms an azeotrope with water, meaning it cannot be further concentrated by simple distillation.
Hydroiodic Acid vs. Other Hydrogen Halides
| Property | Hydroiodic Acid (HI) | Hydrobromic Acid (HBr) | Hydrochloric Acid (HCl) |
|---|---|---|---|
| Acid Strength (pKa) | -9.3 | -8.7 | -6.3 |
| Boiling Point (°C) | -35.36 | -66.8 | -85.05 |
| Reducing Power | High | Moderate | Low |
| Industrial Use | Reducing agent, organic synthesis | Bromide production, organic synthesis | Metal cleaning, pH control |
Uses of Hydroiodic Acid
Curious about the uses of sulfurous acid? Eureka Technical Q&A provides expert insights into its applications in food preservation, water treatment, and industrial processes, helping you understand its benefits and safe handling practices.
1. Industrial Applications
- Reducing Agent: Used in chemical industries to facilitate reduction reactions.
- Iodide Production: Serves as a key precursor in manufacturing iodide salts for pharmaceuticals and photography.
2. Laboratory and Chemical Research
- Organic Synthesis: Used in iodination reactions to introduce iodine atoms into organic molecules.
- Reductive Cleavage: Helps break carbon-oxygen bonds, such as converting epoxides into alcohols.
3. Medical and Pharmaceutical Use
- Disinfectant Production: Used in the manufacture of iodophors, a key ingredient in antiseptics.
Hazards and Safety Considerations
1. Reactivity and Explosion Risks
- Reacts violently with strong oxidizing agents, leading to the release of iodine.
- Can react with metals, producing flammable hydrogen gas, which poses explosion risks.
2. Corrosive Effects
- Causes severe burns and irritation upon skin or eye contact.
- Inhalation of vapors may lead to respiratory tract damage and breathing difficulties.
3. Environmental Impact
- Can cause soil and water contamination if not disposed of properly.
- Iodine emissions from HI can contribute to environmental pollution.
Application Cases
| Product/Project | Technical Outcomes | Application Scenarios |
|---|---|---|
| Anhydrous HI Production System Honeywell International Technologies Ltd. | Achieves low water concentrations in HI production using nickel(II) iodide, activated alumina, and azeotropic distillation | Industrial-scale production of high-purity anhydrous hydrogen iodide for chemical manufacturing |
| Synthetic Acid Composition Fluid Energy Group Ltd. | Replaces hydrochloric acid with a urea-HCl mixture, incorporating metal iodide for enhanced performance | Oil and gas industry activities requiring large amounts of acid, such as well stimulation and scale removal |
| Electrochemical Carbohydrate Converter The Regents of the University of California | Efficiently converts carbohydrates to hydrocarbons using electrochemically regenerated HI | Biomass conversion for renewable fuel production |
| ITO Etching Solution Canon, Inc. | Utilizes HI and ferric chloride mixture for precise etching of ITO, with efficient regeneration method | Manufacturing of liquid crystal display devices requiring minute electrode patterns |
| CsPbI3 Perovskite Solar Cells The University of New South Wales | Achieves 6.44% efficiency using optimized HI concentration of 36 μL/mL in perovskite formation | Development of high-efficiency perovskite/silicon tandem solar cells |
Safe Handling and Storage Practices
1. Personal Protective Equipment (PPE)
- Eye Protection: Wear safety goggles or face shields to prevent eye exposure.
- Skin Protection: Use acid-resistant gloves and protective clothing to avoid contact.
- Respiratory Protection: Work in well-ventilated areas or use appropriate respirators.
2. Engineering Controls
- Conduct all operations in fume hoods to minimize inhalation risks.
- Use spill containment measures to manage accidental leaks.
3. Storage Guidelines
- Store in tightly sealed, corrosion-resistant containers away from oxidizing agents and metals.
- Keep storage areas cool, dry, and well-ventilated.
4. Emergency Procedures
- Spill Response: Neutralize small spills with a weak base such as sodium bicarbonate and clean with absorbent materials.
- First Aid Measures: If skin or eye contact occurs, rinse immediately with plenty of water for at least 15 minutes and seek medical attention.
FAQs About Hydroiodic Acid
1. What is hydroiodic acid used for?
Chemists primarily use hydroiodic acid as a reducing agent in chemical synthesis, for iodide production, and in organic iodination reactions.
2. Why is hydroiodic acid considered dangerous?
Its strong acidity and high reactivity make it highly corrosive and hazardous, capable of causing severe burns and dangerous chemical reactions.
3. How should hydroiodic acid be stored safely?
Store it in tightly sealed, corrosion-resistant containers in cool, well-ventilated areas, away from incompatible substances.
4. Can hydroiodic acid cause burns?
Yes, it is highly corrosive and can cause severe burns upon skin or eye contact. Proper PPE should always be used when handling it.
5. How do you neutralize a hydroiodic acid spill?
Spills should be neutralized with a weak base such as sodium bicarbonate, followed by cleaning with absorbent materials and thorough rinsing.
Conclusion
Hydroiodic acid is an essential chemical in industrial and laboratory settings, primarily used as a strong acid and reducing agent. However, its corrosive nature and hazardous reactivity necessitate strict safety protocols for handling, storage, and disposal. Understanding its properties and risks allows for responsible and efficient use in various scientific and industrial applications.
To get detailed scientific explanations of Hydroiodic Acid, try Patsnap Eureka.


