Overview
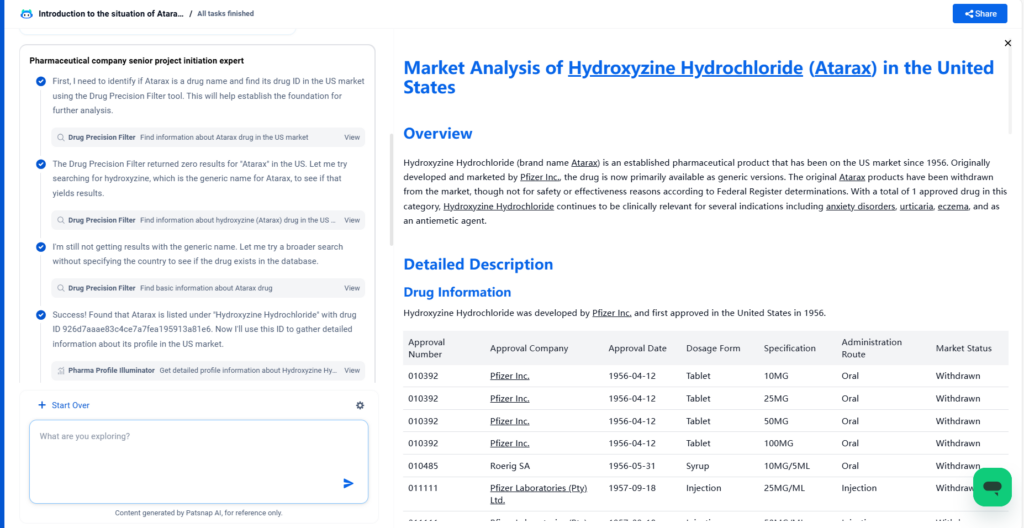
Hydroxyzine Hydrochloride (brand name Atarax) is an established pharmaceutical product that has been on the US market since 1956. Originally developed and marketed by Pfizer Inc., the drug is now primarily available as generic versions. The original Atarax products have been withdrawn from the market, though not for safety or effectiveness reasons according to Federal Register determinations. With a total of 1 approved drug in this category, Hydroxyzine Hydrochloride continues to be clinically relevant for several indications including anxiety disorders, urticaria, eczema, and as an antiemetic agent.
Detailed Description
Drug Information
Hydroxyzine Hydrochloride was developed by Pfizer Inc. and first approved in the United States in 1956.
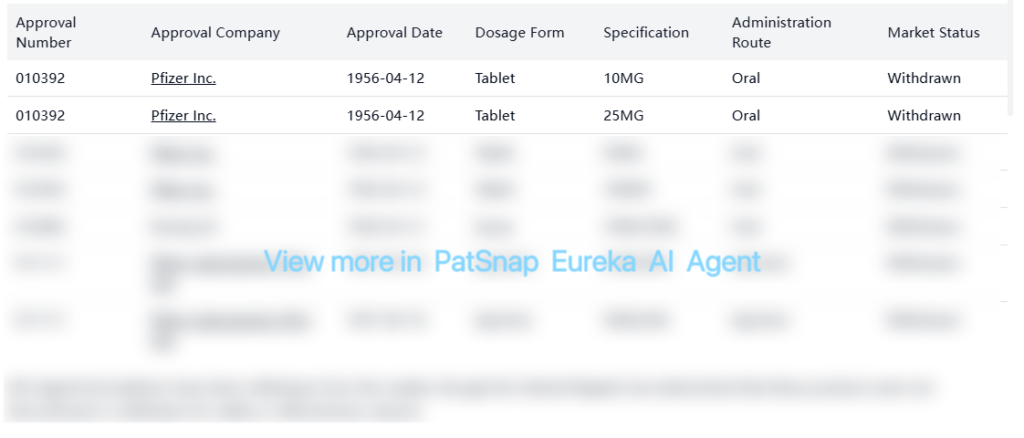
All original formulations have been withdrawn from the market, though the Federal Register has determined that these products were not discontinued or withdrawn for safety or effectiveness reasons.
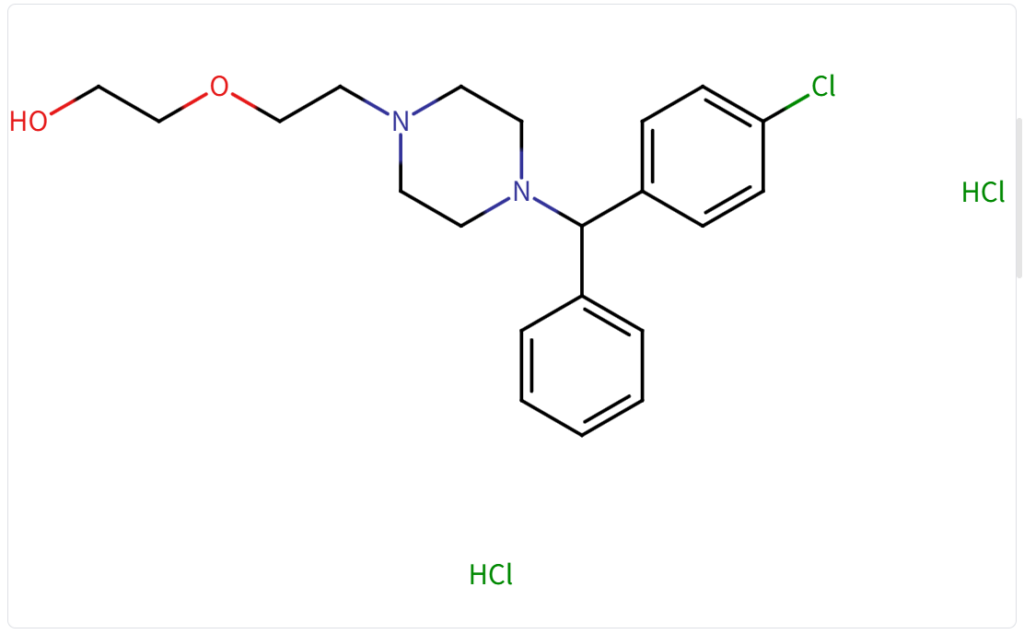
Structure
Patent Barrier Analysis
Registration Patent Analysis
No core patents were found for Hydroxyzine Hydrochloride in the FDA Orange Book, indicating that the compound is off-patent and available for generic manufacturing without patent barriers.
Other Original Patent Analysis
| Patent Number | Simple Legal Status | Application Date | Estimated Expiry | Patent Type | Applicant |
|---|---|---|---|---|---|
| IL52588A | Inactive | 1977-07-25 | N/A | Formulation | Pfizer Inc. |
This formulation patent from Pfizer is inactive and relates to pharmaceutical compositions combining pirbuterol and hydroxyzine as bronchodilators.
Other Non-Original Patent Analysis
The patent landscape shows primarily inactive patents, with one pending application from Korea Research Institute of Chemical Technology for a new use as an antiviral composition. This pending patent is unlikely to affect the current generic status of the base compound but could potentially impact specific new applications if approved.
Clinical Results
Based on FDA label clinical insights, Hydroxyzine Hydrochloride has demonstrated several pharmacological effects:
- Skeletal Muscle Relaxation: Experimental studies have demonstrated that hydroxyzine hydrochloride produces primary skeletal muscle relaxation, suggesting a central nervous system component to the drug’s mechanism of action.
- Bronchodilator Activity: Hydroxyzine has shown bronchodilation effects, likely related to its central suppression of subcortical activity, which might help alleviate histamine-mediated bronchoconstriction.
- Antihistaminic and Analgesic Effects: The drug exhibits measurable antihistaminic activity and also provides analgesia. Both these pharmacologic actions have been demonstrated in laboratory settings and further confirmed through clinical usage.
- Antiemetic Activity: Hydroxyzine hydrochloride’s antiemetic properties were evaluated using specific tests:
- The Apomorphine TestThe Veriloid Test
These effects have been confirmed through both experimental studies and clinical evaluations, establishing Hydroxyzine Hydrochloride’s multifaceted pharmacological profile.
Infringement Cases
No patent infringement incidents specifically involving Hydroxyzine Hydrochloride have been reported or documented in the available references.
Policy and Regulatory Risk Warning
After a comprehensive search, Hydroxyzine Hydrochloride (Atarax) has no market exclusivity or data protection period in the United States. The original approvals date back to 1956-1957, and all exclusivity periods have long expired.
Market Entry Assessment & Recommendations
Based on the comprehensive analysis, Hydroxyzine Hydrochloride presents a very low-risk opportunity for generic manufacturers in the US market:
- Patent Landscape: With no active core patents and most secondary patents expired, there are minimal patent barriers to market entry. The only pending patent is for a new use (antiviral) that would not affect traditional applications.
- Regulatory Status: All original Pfizer products are withdrawn but not for safety or efficacy reasons, indicating the regulatory pathway for generics remains clear.
- Market Positioning Strategies:
- Focus on cost optimization and manufacturing efficiency to compete in this mature generic market
- Consider developing improved formulations or delivery systems (e.g., extended-release as suggested by Dr. Reddy’s patent application) to differentiate from existing generics
- Explore combination products leveraging hydroxyzine’s multiple mechanisms of action (antihistamine, anxiolytic, antiemetic)
- Investigate emerging applications such as the potential antiviral use being researched by Korea Research Institute of Chemical Technology
- Market Access Considerations:
- Ensure competitive pricing to gain access to formularies and procurement contracts
- Highlight the multi-functional nature of the drug (antihistamine, anxiolytic, antiemetic) in marketing materials
- Target hospital formularies where the drug’s multiple uses can provide inventory efficiency
- Differentiation Opportunities:
- Develop patient-friendly formulations (taste-masked liquids, orally disintegrating tablets)
- Consider pediatric-specific formulations and dosing, as hydroxyzine is often used in children
- Explore novel delivery systems that might improve onset of action or reduce sedative side effects
The market for Hydroxyzine Hydrochloride is mature but stable, with continued clinical utility across multiple indications making it a low-risk, steady-return opportunity for generic manufacturers.
For more detailed information of Hydroxyzine Hydrochloride(Atarax), try PatSnap Eureka AI Agent.