
Lubiprostone is a selective chloride channel activator that represents a class of targeted therapies for chronic gastrointestinal motility disorders. Approved for chronic idiopathic constipation (CIC), opioid-induced constipation (OIC), and irritable bowel syndrome with constipation (IBS-C), it offers symptom relief by enhancing intestinal fluid secretion without systemic absorption.
This article explores the pharmacological profile, dosage strategy, clinical application, and market evolution of lubiprostone. With PatSnap Eureka AI Agent, clinicians and pharmaceutical researchers can access real-time insights into lubiprostone’s clinical trials, global regulatory submissions, pharmacological pathways, and comparative market data—providing a 360° view of its therapeutic potential.
What is Lubiprostone?
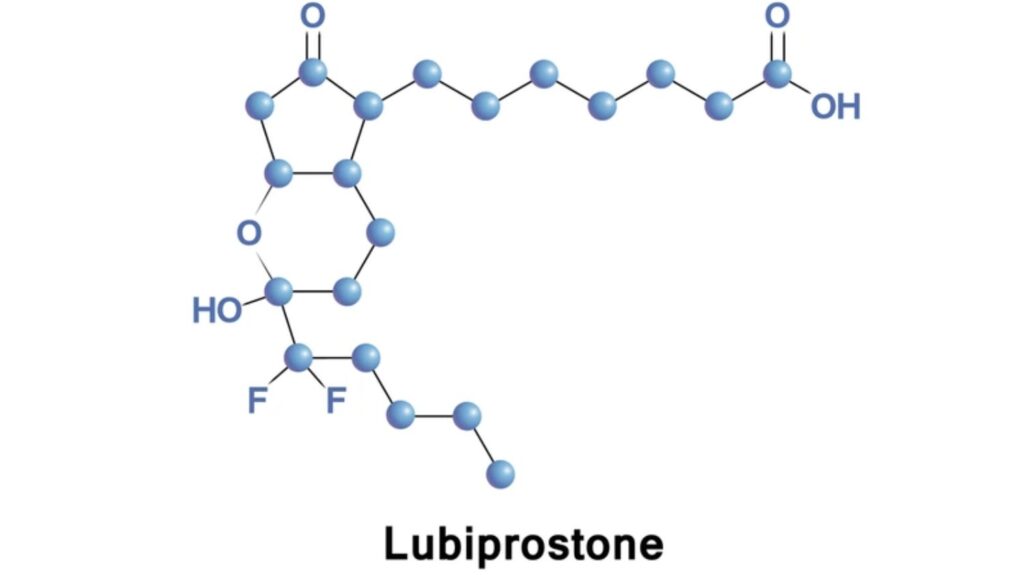
Lubiprostone is a locally acting chloride channel (ClC-2) activator approved by the FDA for the treatment of CIC, IBS-C in adult women, and OIC in adults with chronic non-cancer pain. It promotes intestinal motility by increasing chloride-rich fluid secretion into the intestinal lumen, thus softening stool and facilitating passage.
It is marketed under the brand name Amitiza and is one of the few FDA-approved agents with a mechanism focused on fluid secretion rather than muscular stimulation, distinguishing it from stimulant laxatives and other prokinetic agents.
Key Characteristics of Lubiprostone
| Feature | Description |
|---|---|
| Drug Class | Chloride Channel Activator (ClC-2) |
| Brand Name | Amitiza |
| Approval Year | 2006 (FDA) |
| Indications | CIC, OIC (non-cancer pain), IBS-C (adult women) |
| Site of Action | Intestinal epithelial cells (luminal-facing ClC-2 channels) |
| Systemic Absorption | Minimal (acts locally in the GI tract) |
| Formulation | Oral soft-gel capsule |
Mechanism of Action
Lubiprostone activates ClC-2 chloride channels on the apical membrane of intestinal epithelial cells. This action promotes chloride ion efflux, which drives passive movement of sodium and water into the intestinal lumen. The resulting fluid secretion softens stool and improves intestinal transit without disrupting electrolyte balance.
- ClC‑2 Channel Activation
Lubiprostone targets ClC‑2 channels with high specificity. Its action does not rely on protein kinase A. Immunoconfocal microscopy places ClC‑2 on the apical membrane. Short‑circuit current studies show that lubiprostone—but not vehicle—raises chloride flow. Patch‑clamp tests confirm the signal: lubiprostone increases Cl⁻ currents in ClC‑2‑transfected cells in a dose‑dependent manner. It fails to boost conductance in CFTR‑transfected cells, underscoring selectivity.
- Intestinal Fluid Secretion and Motility
By pulling chloride, sodium, and water into the lumen, lubiprostone loosens stool and speeds transit. It accelerates total colonic transit yet leaves ascending‑colon emptying unchanged. Imaging studies show clearer small‑bowel views and a shorter small‑bowel transit time (SBTT). In vitro, the drug boosts EFS‑induced contractions in circular smooth muscle and raises pyloric sphincter tone; it does not affect longitudinal muscle. An EP1 antagonist blocks these excitatory effects, pointing to prostaglandin involvement.
- Prostaglandin Receptor Engagement
Lubiprostone activates PGE receptors, with EP4 as the primary target. Sheep‑airway data and multiple GI studies link EP4 activation to anion secretion and ClC‑2/CFTR channel opening.
- Clinical Effects
Clinically, lubiprostone improves chronic idiopathic constipation and IBS‑C. Patients pass stools more often, see softer consistency, and strain less. A meta‑analysis notes nausea, vomiting, and diarrhea as the most common adverse events (incidence 2.4–75 %). Rapid fluid influx into the small intestine likely drives these reactions.
Side Effects and Safety
Lubiprostone is generally well tolerated. Common side effects include:
- Nausea (most frequent)
- Diarrhea
- Headache
- Abdominal distension or pain
Serious adverse effects are rare. Due to minimal systemic absorption, the drug has no known cardiovascular, renal, or CNS toxicity.
Pregnancy Category C: Avoid use unless absolutely necessary. Animal studies have shown fetal loss at high doses.
Comparison with Other Constipation Treatments
Market Competition of Lubiprostone
Lubiprostone faces competition from other medications within the same therapeutic class (prosecretory agents) and from medications in different classes that are also used to treat constipation.
- Prosecretory Agents: The primary competitor in this class is linaclotide (Linzess®), which acts via a different mechanism (guanylate cyclase-C receptor activation). Linaclotide is approved for both CIC and IBS-C.
- Other Constipation Medications: Lubiprostone competes with a wide range of laxatives, including over-the-counter products, for the treatment of constipation. These include bulk-forming agents, osmotic laxatives, stimulant laxatives, and stool softeners.
| Drug | Class | Main Use | Key Advantage | Limitations |
|---|---|---|---|---|
| Lubiprostone | ClC-2 Activator | CIC, OIC, IBS-C | Local action, minimal systemic absorption | Nausea, cost |
| Linaclotide | GC-C Agonist | CIC, IBS-C | Improves bloating, stool frequency | Diarrhea, not approved for OIC |
| Prucalopride | 5-HT₄ Receptor Agonist | CIC | Stimulates colonic motility | Headache, abdominal pain |
| Tenapanor | NHE3 Inhibitor | IBS-C | Reduces sodium absorption, softens stool | Not approved for CIC or OIC |
Differentiation:
Lubiprostone differentiates itself in the market through several key factors:
- Novel Mechanism of Action: Lubiprostone’s activation of ClC-2 channels is a unique mechanism among prescription constipation medications, offering an alternative for patients who do not respond to other therapies.
- Targeted Indications: Lubiprostone is specifically approved for CIC, IBS-C, and OIC, allowing it to address the needs of patients with these specific conditions.
- Safety and Tolerability: Lubiprostone is generally well-tolerated, with the most common side effect being nausea. In a retrospective cohort study comparing lubiprostone and elobixibat (another constipation medication) in Japanese patients, no significant difference in the hazard ratio for common events, including drug add-ons, was found.
- Formulation and Administration: Lubiprostone is available as a soft gelatin capsule, taken orally with food. The dosage is 24 µg twice daily for CIC and IBS-C, and 24 µg twice daily for OIC. A stable pharmaceutical formulation comprising lubiprostone and at least one propylene glycol ester has been developed, which provides better stability and solubility.
Lubiprostone occupies a unique position in the constipation treatment landscape due to its distinctive mechanism of action, targeted indications, and favorable safety profile. While it faces competition from other constipation medications, its ability to address specific patient populations and provide an alternative to conventional therapies contributes to its market differentiation. The development of stable pharmaceutical formulations further enhances its potential.
Conclusion
Lubiprostone remains a valuable asset in the treatment of chronic functional constipation and IBS-C, especially for patients who do not respond to bulk-forming or stimulant laxatives. Its local mechanism, low systemic exposure, and broad clinical utility distinguish it in the growing market of prosecretory agents.
As the GI therapeutic landscape evolves, tools like PatSnap Eureka AI Agent provide unmatched visibility into the mechanisms, trials, safety data, and market positioning of lubiprostone and its emerging competitors. For R&D teams, formulators, and commercial strategists, Eureka offers a comprehensive lens through which to optimize development decisions and maximize patient outcomes.
FAQs
It’s not a traditional laxative. Lubiprostone is a chloride channel activator that increases intestinal fluid secretion to improve motility.
Current FDA approval is limited to adult women for IBS-C. Use in men remains off-label.
Unlike other agents, lubiprostone works locally by activating chloride channels and doesn’t significantly affect systemic signaling pathways.
Use PatSnap Eureka AI Agent to access real-time regulatory filings, clinical trials, and pharmacological intelligence related to lubiprostone and competing GI therapies.
For a deeper understanding of Lubiprostone, try PatSnap Eureka AI Agent, which provides detailed intelligence on global clinical trials, pharmacodynamics, regulatory filings. Moreover, it can also provide market activity related to Lubiprostone and other chloride channel modulators used in gastrointestinal therapies.

