Magnesium iron silicate hydroxide, often represented by minerals such as cummingtonite, anthophyllite, and serpentine, holds growing attention due to its multifunctional properties. This natural mineral combines magnesium, iron, silicon, oxygen, and hydroxide in a robust crystal matrix, making it useful across environmental, construction, and filtration industries. In this post, we explore its composition, grades, performance, cross‑industry applications, and emerging innovations, giving technical professionals a comprehensive understanding of its potential through PatSnap Eureka AI Agent.
Material Composition and Grade Data
Magnesium iron silicate hydroxide typically has the chemical formula (Mg,Fe)₃Si₂O₅(OH)₄. It belongs to the serpentine group of minerals and exists in polymorphic forms like lizardite, antigorite, and chrysotile. These forms vary in their crystalline habits—some appearing in sheeted structures and others in fibrous or platy morphologies.
Magnesium iron silicate hydroxide follows a general formula:
(Mg,Fe)_m Si_n O_x (OH)_y, where typical phases include:
- Cummingtonite/Grunerite series: (Mg,Fe)_7Si_8O_22(OH)_2
- Serpentine group: (Mg,Fe)_3Si_2O_5(OH)_4
Major suppliers include geological assay streams from USGS, Mindat, and Chemeurope—citing grade data:
| Mineral Phase | Mg/(Mg+Fe) Ratio | Supplier / Standard | Application Use |
|---|---|---|---|
| Magnesiocummingtonite | >0.70 | Mindat, UK sources | Insulation, ceramics |
| Cummingtonite (Fe-rich) | 0.30–0.70 | USGS, metallogenic mapping | Filtration, CO₂ sequestration |
| Serpentine (various polymorphs) | – | Chemeurope, museum data | Brake linings, adsorbents |
These phases deliver variations in density (3.1–3.6 g/cm³), hardness (Mohs 5–6), and thermal expansion—all critical attributes for downstream industrial performance
Key Properties That Define the Material
- Thermal resilience (up to ~600 °C) and low thermal expansion, making it suitable for insulation and heat-resistant applications.
- Moderate density (~3.1–3.6 g/cm³) supports structural use without excessive weight.
- Layered crystal structure of hydroxide-silicate enables chemical adsorption and tuneable ion exchange.
- Chemical stability across neutral–alkaline environments renders it fitting for filtration and water treatment.
- Mechanical hardness (5–6 Mohs) ensures moderate abrasion and wear resistance.

Core Applications Across Industries
Geosciences and Petrology
Magnesium iron silicate hydroxide is central to studies of ultramafic rocks and mantle mineralogy. Its transformation behavior under high-pressure and high-temperature conditions helps geologists model subduction zones and Earth’s deep carbon cycle. In tectonophysics, it’s used to simulate serpentinization processes that influence plate dynamics and fluid transport in oceanic crusts.
Environmental Remediation
Due to its ability to bind heavy metals and its alkaline buffering capacity, magnesium iron silicate hydroxide has been explored as a low-cost, eco-friendly material for soil remediation and acid mine drainage treatment. Its high surface area and reactivity allow it to immobilize pollutants such as Pb²⁺, Cd²⁺, and As⁵⁺ through ion exchange and surface precipitation mechanisms.
Carbon Sequestration and Mineralization
One of the most promising uses of magnesium iron silicate hydroxide is in carbon capture and storage (CCS). The mineral readily reacts with CO₂ to form stable magnesium carbonate phases, offering a pathway for permanent CO₂ mineralization. This process mimics natural weathering and has been tested in enhanced weathering and direct air capture systems.
Refractory and Ceramic Materials
The high thermal stability of magnesium iron silicate hydroxide makes it suitable for use in refractory linings and ceramics. Modified synthetic analogs have been used in producing heat-resistant bricks, fiber composites, and insulating materials for industrial furnaces. Its fibrous forms are also being investigated as safer alternatives to asbestos in thermal insulation.
Construction and Cement Industry
In alkali-activated cement systems, magnesium iron silicate hydroxide can act as a pozzolanic or filler component, enhancing mechanical properties and durability. Research shows its role in reducing CO₂ emissions in green cement formulations by enabling low-lime clinker alternatives.
Comparative Advantages and Limitations
Magnesium iron silicate hydroxide (commonly known as serpentine-group minerals) exhibits a compelling balance of functional properties and structural versatility. Its unique composition and morphology make it suitable for a range of industrial and scientific applications. However, like most naturally derived or composite materials, it comes with both strengths and trade-offs.
Advantages
- Thermal Stability and Fire Resistance
Due to its layered silicate structure and hydroxyl interlayers, magnesium iron silicate hydroxide demonstrates high thermal stability, making it valuable as a flame-retardant additive in polymers and coatings. - Low Density with High Aspect Ratio
This mineral typically exhibits a high aspect ratio when processed into nano-platelets, improving its reinforcing capabilities in polymer matrices without adding significant weight. - Chemical Inertness in Neutral and Alkaline Media
It offers strong resistance to chemical degradation, especially in neutral and mildly alkaline environments, allowing long-term use in chemical processing and containment applications. - Ion Exchange and Adsorption Capacity
Its ability to capture heavy metals and pollutants—thanks to its charged lamellar surfaces—makes it highly promising in environmental remediation and wastewater treatment. - Biocompatibility and Low Toxicity
Certain forms of magnesium iron silicate hydroxide (particularly purified or synthetic analogs) exhibit low toxicity and are being explored for biomedical scaffolds, wound dressings, and drug carriers. - Natural Abundance and Low Cost
As a mineral derived from serpentinized ultramafic rocks, it is relatively abundant and cost-effective, reducing raw material expenses for large-scale industrial use.
Limitations
- Moisture Sensitivity and Structural Breakdown
Exposure to moisture can lead to interlayer swelling, weakening mechanical integrity over time—particularly in high-humidity environments or aqueous dispersions. - Limited Electrical Conductivity
Its insulating properties may restrict usage in advanced electronics or conductive polymer composites unless hybridized with conductive fillers. - Inconsistent Purity in Natural Forms
Naturally sourced magnesium iron silicate hydroxide often contains impurities (e.g., asbestos-like fibers or trace metals), necessitating costly purification for high-performance applications. - Processing Challenges at Nanoscale
While nanoscale versions offer excellent performance, exfoliating or synthesizing them uniformly at scale remains complex and energy-intensive. - Thermal Dehydroxylation at High Temperatures
Beyond certain thresholds (~600–700 °C), the mineral undergoes dehydroxylation and structural collapse, limiting its use in ultra-high temperature processes.
Comparative Overview with Similar Fillers
| Property | Magnesium Iron Silicate Hydroxide | Talc | Kaolinite | Montmorillonite |
|---|---|---|---|---|
| Thermal Stability | High | Medium | Medium | Medium |
| Ion Exchange Capacity | Moderate–High | Low | Low | High |
| Density (g/cm³) | ~2.6 | ~2.7 | ~2.6 | ~2.5 |
| Environmental Safety | Generally Safe | Safe | Safe | Varies |
| Electrical Conductivity | Insulating | Insulating | Insulating | Insulating |
| Cost | Low–Medium | Low | Low | Medium |
| Nanocomposite Compatibility | High (if exfoliated) | Moderate | Moderate | High |
This comparative perspective underscores magnesium iron silicate hydroxide’s strategic position as a multifunctional, safe, and scalable material—particularly when purity control and moisture management are prioritized.
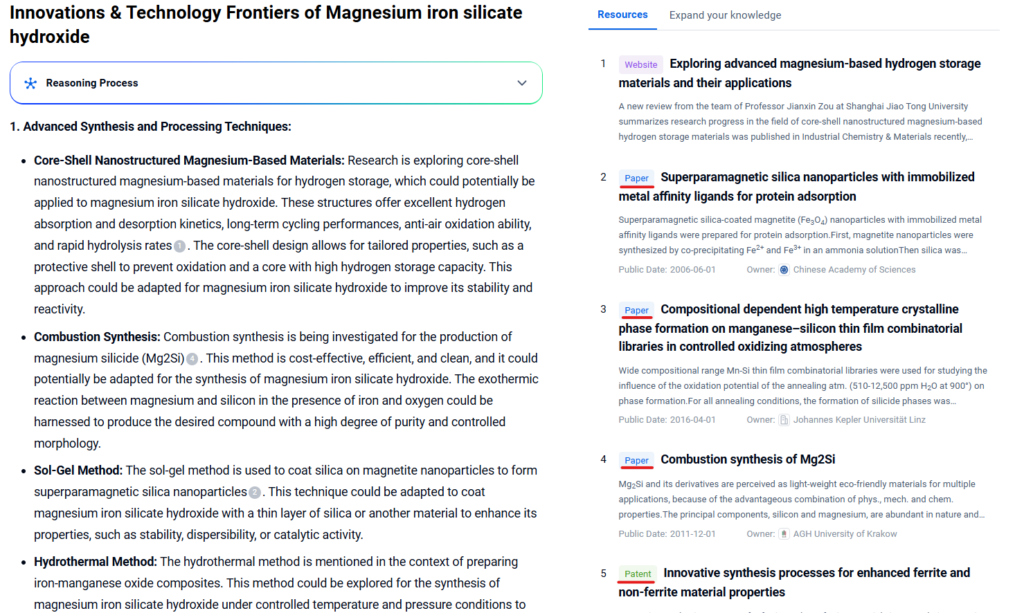
Innovations & Technology Frontiers
- Synthetic Derivatives for CCS: Researchers are synthesizing magnesium iron silicate hydroxide analogs with tailored nanostructures to maximize CO₂ uptake capacity. Advances in hydrothermal synthesis and surface activation are enabling more efficient carbonation at ambient temperatures.
- Nano-structuring for Adsorption Efficiency: Innovative methods using ball milling and surface grafting are enhancing the sorption capacity of magnesium iron silicate hydroxide for removing contaminants from water and soil.
- Biomimetic Weathering for Carbon Capture: Several projects are mimicking natural serpentinization in lab reactors to accelerate mineral carbonation using magnesium iron silicate hydroxide. These systems are being evaluated for scalability in negative emission technologies.
- Green Composite Development: Magnesium iron silicate hydroxide is being explored as a reinforcing filler in bio-based polymer composites, aiming to produce flame-retardant, lightweight structural materials.
- Remote Sensing and Machine Learning in Mapping Deposits: Recent developments in hyperspectral imaging and AI-driven mineral classification are helping locate high-purity serpentine deposits, which are critical for sustainable sourcing in large-scale applications.
Industry Challenges and Future Outlook
As magnesium iron silicate hydroxide adoption expands, these issues demand solutions:
- Health concerns with asbestos-like minerals—require strict sourcing and certification.
- Variability across deposits hinders standardization—industry needs consistent grading and purity.
- Scale-up barriers for nano‑modified or functionalized versions.
- Limited performance at very high temperatures—research aims to extend operational range.
- Regulatory landscape in environmental and construction applications—demands clarity and compliance.
Conclusion
Magnesium iron silicate hydroxide sits at the crossroads of geology, environmental engineering, and sustainable material innovation. Its structural uniqueness, chemical versatility, and eco-compatibility are opening doors in carbon capture, remediation, and next-generation refractories. As material science continues to interface with planetary health and clean tech, magnesium iron silicate hydroxide will remain a focal point of both foundational research and practical deployment.
FAQs
Yes, though care must be taken with its fibrous forms (like chrysotile), which can pose respiratory risks. Proper encapsulation or substitution with platy forms is recommended.
It reacts with CO₂ to form stable magnesium carbonates, offering a permanent solution for storing captured CO₂ through mineralization.
Not entirely, but it can significantly reduce cement usage as a filler or reactive pozzolan in low-carbon binders.
Synthetic forms can be optimized for surface area, purity, and performance in specific applications like water purification or CO₂ capture.
Yes. It is used in certain environmental treatment media, ceramic composites, and experimental carbon capture technologies.
Want to uncover patent trends, supplier landscapes, or R&D breakthroughs in magnesium iron silicate hydroxide applications?
👉 Explore deeper insights using PatSnap Eureka AI Agent—your AI-driven resource for materials intelligence, innovation scouting, and sustainable engineering strategy.