
Malonic ester synthesis is a classic carbon–carbon bond-forming reaction used to create substituted carboxylic acids, especially α-substituted acetic acids. This reaction is widely used in organic synthesis due to its versatility, predictability, and simplicity, making it a go-to method for building complex molecules in pharmaceuticals, agrochemicals, and fine chemicals. This article breaks down the key steps, reaction mechanism, and examples of malonic ester synthesis, helping you understand how this process works and when to use it.
Get Started with PatSnap Eureka AI Agent
Malonic ester, also known as malonic diester, is an organic compound that plays a significant role in various chemical syntheses. It is typically represented by the formula (RCO2CH2CO2R’), where R and R’ are alkyl or aryl groups. The most common form is diethyl malonate, which consists of two ethyl ester groups attached to a malonic acid backbone.
Definition and Structure
Malonic Ester Synthesis – How does it work? Eureka Technical Q&A explains the steps and mechanism behind this classic organic reaction, showing how malonic esters are used to create substituted acetic acids—with clear examples to help you apply it in real synthesis.
Malonic esters are derivatives of malonic acid, which contains two carboxyl groups (-COOH) separated by a methylene group (-CH2-). In ester form, these carboxyl groups are converted to ester groups (-COOR), making the molecule more stable and easier to handle in organic synthesis. The structure of malonic esters contributes to their reactivity, particularly in nucleophilic addition reactions, due to the presence of the activated methylene group (-CH2-) between the two ester groups.
Steps in Malonic Ester Synthesis
- Saponification: This initial step involves the hydrolysis of the malonic ester to form the corresponding carboxylic acid and alcohol. For instance, diethyl malonate can be hydrolyzed to yield malonic acid and ethanol.
- Decarboxylation: In this step, the malonic acid undergoes decarboxylation, typically facilitated by heat, to form a carbon dioxide molecule and a one-carbon homologated product. This reaction is crucial for extending the carbon chain in the target molecule.
- Tautomerization: Following decarboxylation, tautomerization occurs, where the enol form of the intermediate is converted into the more stable keto form. This step is essential for the subsequent reactions, particularly in forming amides and esters.
Image Source: Wikipedia

Mechanism of Malonic Ester Synthesis
The mechanism involves several key reactions:
- Enolate Formation: The malonic ester is deprotonated to form an enolate ion, which is a nucleophilic species capable of undergoing further reactions.
- Alkylation: The enolate ion can then be alkylated at the alpha carbon, allowing for the introduction of various substituents. This step is crucial for the synthesis of diverse amide and ester compounds.
- Hydrolysis and Decarboxylation: The alkylated intermediate undergoes hydrolysis to form the corresponding carboxylic acid, which can then be decarboxylated to yield the final product with an extended carbon chain.
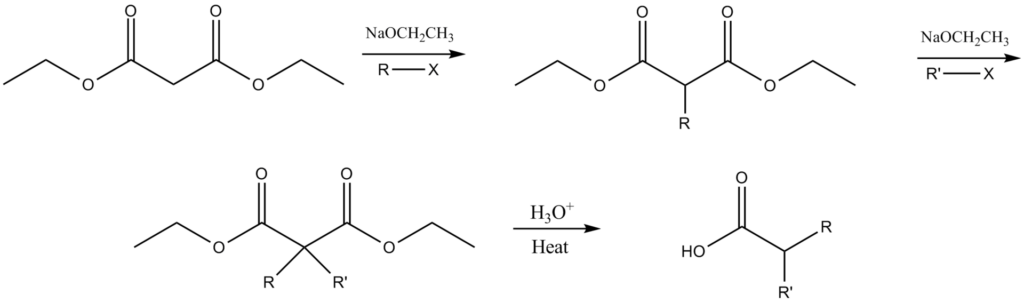
Examples of Malonic Ester Synthesis
- Synthesis of Amides: Malonic ester synthesis can be used to create amides by reacting the alkylated intermediate with amines. For example, the synthesis of N-substituted amides involves the reaction of a malonic ester with an amine, followed by decarboxylation and tautomerization to form the amide bond.
- Synthesis of Ester Compounds: Malonic ester synthesis can also be employed to create ester compounds by reacting the alkylated intermediate with alcohols. This method is useful in producing various ester derivatives with specific functional groups.
- One-Pot Synthesis: A ‘one-pot’ synthesis approach has been developed for the efficient production of α-1,2,4-oxadiazolo esters from malonic diesters and amidoximes under solvent-free conditions. This method highlights the versatility and efficiency of malonic ester synthesis in creating complex molecular structures.
Applications and Significance
- Synthesis of Pharmaceuticals: Malonic ester is widely used in the synthesis of pharmaceuticals. Its ability to act as a building block allows for the creation of complex molecules. For instance, it can be used to synthesize malonamic acid esters, which are intermediates in the production of certain pharmaceuticals.
- Antioxidants in Diesel Fuel: Malonic acid esters have been developed as antioxidants for diesel fuel. These compounds help improve the stability and performance of diesel fuel by preventing oxidation and degradation.
- Catalysis: Malonic ester synthesis has been improved using novel catalysts such as tungsten oxide nanoparticles. These catalysts enhance the efficiency and yield of malonic ester production through methods like ozonolysis.
- Amide Synthesis: Malonic ester is employed in the synthesis of amides, which are crucial components in various chemical and pharmaceutical applications. This is achieved through decarboxylative acylation reactions that allow for the formation of amides with diverse substituents.
- Labelled Fatty Acid Synthesis: Malonic ester is used in the preparation of labelled fatty acids. This application is beneficial for research and medical diagnostics, where labelled compounds are essential for tracing biochemical pathways.
- Remediating Wax Buildup: Olefinic ester compositions derived from malonic ester derivatives are used to remove wax buildup in oil and gas-related applications. This helps in maintaining the efficiency of pipelines and other equipment.
- Asymmetric Synthesis: Malonic ester derivatives are utilized in asymmetric synthesis, which is crucial for producing enantiomerically pure compounds. This is particularly important in the pharmaceutical industry for drugs that require specific stereochemistry.
In summary, malonic ester synthesis is a powerful technique in organic chemistry, involving key steps of saponification, decarboxylation, and tautomerization. It enables the creation of diverse amide and ester compounds, making it invaluable in various scientific and industrial domains.
Application Cases
| Product/Project | Technical Outcomes | Application Scenarios |
|---|---|---|
| Malonic Acid Dialkyl Ester Production Dynamit Nobel AG | Efficient production of malonic acid dialkyl esters using cobalt catalysts and controlled pH, achieving high yields and environmental sustainability. | Pharmaceutical and chemical industries requiring efficient and eco-friendly synthesis of malonic acid dialkyl esters. |
| Tungsten Oxide Nanoparticle Catalyst University of Kebangsaan Malaysia | Improved malonic acid ester synthesis via ozonolysis, achieving 10% yield in 2 hours with recyclable catalyst. | Chemical laboratories and industries requiring efficient and sustainable malonic acid ester production. |
| Trifluoromethylmalonic Acid Ester Synthesis Sagami Chemical Research Center | Efficient production of trifluoromethylmalonic acid ester using trifluoromethyl iodide and iron compound catalyst. | Pharmaceutical industry for the synthesis of fluorine-containing drug precursors. |
| Setrobuvir Precursor Synthesis F. Hoffmann-La Roche Ltd. | Novel synthesis of N-(4-methanesulfonylamino-2-sulfamoyl-phenyl)-malonamic acid methyl ester with improved purity and reduced impurities. | Pharmaceutical manufacturing, specifically for the production of Setrobuvir and related compounds. |
| Asymmetric Malonic Acid Bisanilide Synthesis Sandoz AG | Simplified two-step process for producing asymmetric malonic acid bisanilides with high yields and purity, eliminating corrosive intermediates. | Pharmaceutical and fine chemical industries requiring efficient synthesis of complex amide compounds. |
How PatSnap Eureka Accelerates Malonic Ester Synthesis Innovation
Malonic ester synthesis is a cornerstone reaction in organic chemistry, widely used for constructing substituted acetic acids, pharmaceuticals, and fine chemicals. To stay competitive in this space, researchers need rapid access to innovation trends, reaction optimization techniques, and industrial applications. PatSnap Eureka delivers exactly that—equipping R&D teams with real-time intelligence and strategic insight.
- Patent Intelligence: Eureka scans global patent databases to surface the latest developments in malonic ester synthesis, including novel catalysts, reaction conditions, and downstream transformations—streamlining discovery and minimizing duplication.
- Competitive Landscape Tracking: Explore how pharmaceutical, agrochemical, and specialty chemical companies are applying malonic ester synthesis in scalable, cost-effective processes and new product development.
- Trend Forecasting: With AI-powered analytics, Eureka highlights growing areas of demand for malonic ester derivatives—such as in active pharmaceutical ingredients (APIs) and green chemistry—guiding future R&D focus.
- Technical Clustering: Visual clustering tools identify innovation hotspots related to malonic ester , from enantioselective reactions to continuous-flow methodologies, helping teams pinpoint high-impact opportunities.
Whether advancing lab-scale reactions or scaling industrial synthesis, Eureka enables more informed, efficient, and forward-looking innovation in malonic ester synthesis.
To get detailed scientific explanations of methyl calcium nitride, try Patsnap Eureka.


