
TPE Materials Medical Applications Overview
TPE Materials (Thermoplastic Elastomer) combine the properties of thermoplastics and elastomers, offering biocompatibility, sterilizability, and resistance to chemicals and environmental conditions. Their growing use in medical devices, implants, tubing, and packaging is due to their flexibility, durability, and ease of processing. Key applications include:
- Medical Tubing and Catheters: Provide flexibility and kink resistance for smooth insertion and navigation.
- Medical Device Components and Implants: Molded into complex shapes with high precision, biocompatible, and resistant to degradation.
- Medical Packaging and Sterilization: Chemical resistance and ability to withstand high temperatures and pressures.
To get a detailed scientific explanations of tpe, try Eureka.
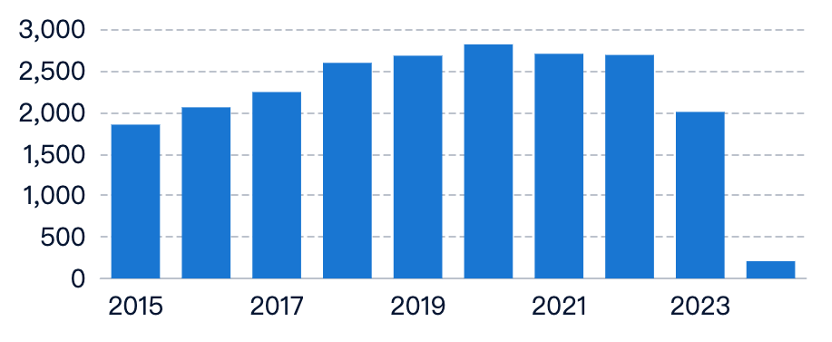
Market Demand for Medical Elastomers
The demand for medical elastomers is growing due to their biocompatibility, flexibility, and durability. Drivers include:
- Aging Population and Chronic Diseases: Increased need for medical devices and implants.
- Orthopedic and Cardiovascular Applications: Used in braces, prosthetics, catheters, and stents.
- Wound Care and Drug Delivery: Used in dressings, bandages, and transdermal systems.
- Minimally Invasive Procedures: Demand for endoscopic instruments, surgical tubing, and seals.
Current State and Challenges of Medical Elastomers
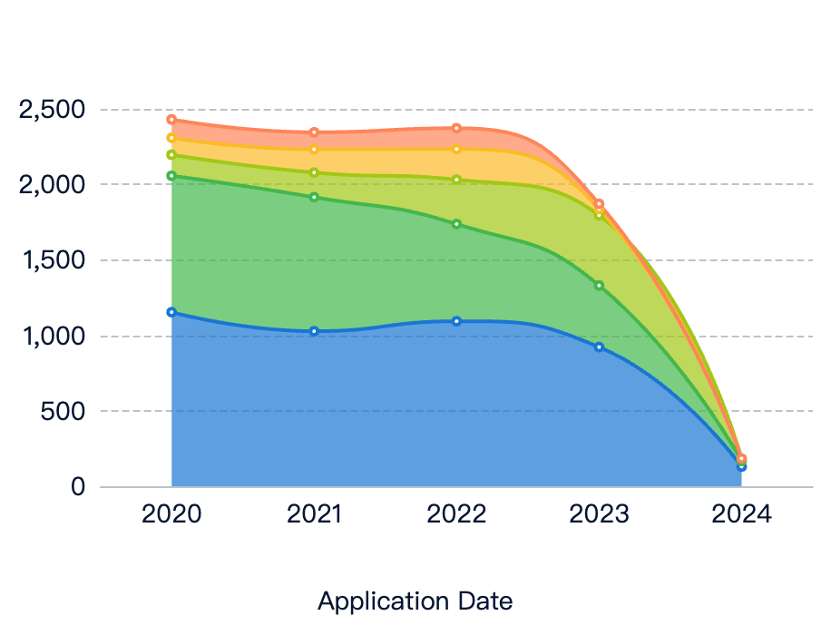
Despite advancements, challenges remain:
- Regulatory Requirements: Stringent biocompatibility standards and extensive testing.
- Mechanical Properties: Need for improved durability under harsh conditions.
- Antimicrobial Properties: Preventing bacterial adhesion and biofilm formation.
- Geographical Distribution: Uneven technology access in developing countries.
Evolution of Thermoplastic Elastomer Technologies
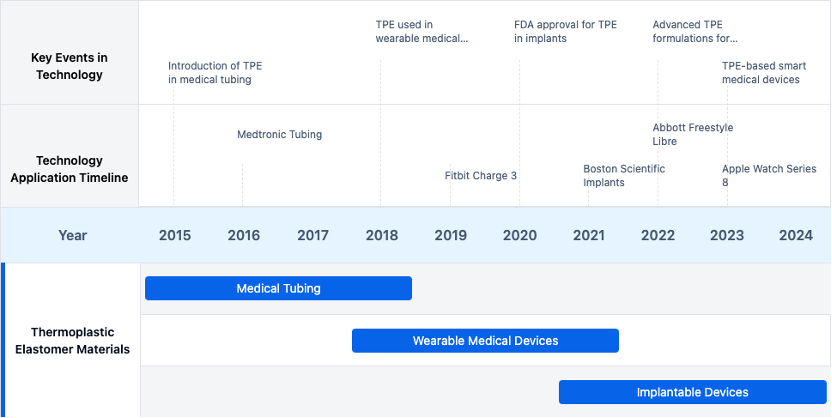
Existing Solutions for Medical Elastomer Applications
TPE Materials for Specific Applications
TPE materials are formulated for various specific applications including:
- Medical Supplies: Infusion tubes, catheters, sealing gaskets with biocompatibility and resistance to bacterial growth.
- Automotive Components: Interior trims, sealing strips, non-pneumatic tires with properties like low odor and high wear resistance.
- High-Performance Applications: Enhanced properties like high temperature resistance, high elasticity, wear resistance, and scratch resistance.
Heat-Resistant and High-Temperature TPE Materials
- High Temperature Resistance: Suitable for applications involving exposure to high temperatures.
- Preparation Methods: Specific formulations, compounding techniques, or processing conditions to enhance thermal stability.
Conductive and Dielectric TPE Materials
- Electrical Properties: Formulated for high conductivity or low dielectric constant for applications in electronics and electrical components.
Antibacterial and Functional TPE Materials
- Antibacterial Properties: Formulated with specific additives for applications requiring antimicrobial characteristics.
TPE Materials with Improved Mechanical Properties
- Enhanced Mechanical Properties: High elasticity, wear resistance, fatigue resistance for durability in demanding applications.
TPE Composite and Blend Materials
- Composites and Blends: Incorporate fillers, reinforcements, or other polymers to achieve desired characteristics.
Key Players in Medical Elastomer Industry
Société de Technologie Michelin
- Focus: High-performance TPEs with enhanced durability, heat resistance, and dynamic properties for automotive and potential medical applications.
Bridgestone Corp.
- Research: TPEs with improved mechanical properties, chemical resistance, and processability for automotive and medical devices.
University of Akron
- Research: Novel TPE compositions, synthesis methods, and structure-property relationships for biocompatible and shape memory TPEs.
Indian Institute of Technology Kharagpur
- Research: TPE blends and nanocomposites with enhanced properties for antimicrobial devices, radiation-resistant equipment, and medical tubing.
ResMed Pty Ltd.
- Patents: TPE compositions and components for medical devices like masks, tubing, and connectors.
Core Innovations in Medical Elastomer Technologies
Patent 1: Low-Odor High-Flame-Retarding Thermoplastic Elastomer TPE Material
- Core Invention Points:
- Halogen-free phosphorus-nitrogen flame retardant for high flame retardancy.
- Organic montmorillonite and metal oxides as deodorants to reduce odor and improve flame retardancy.
- Specific ratio of antioxidants to enhance thermal stability and reduce odor.

Patent 2: Preparation Method of TPE Alloy Material for PCTG Bonding and Encapsulating
- Core Invention Points:
- TPE alloy material with thermoplastic elastomer, plasticizer, engineering plastics, compatibilizer, fillers, and additives for excellent adhesion and performance.
- Preparation method involves pretreatment, mixing, and extrusion through a twin-screw extruder.

Paper 1: Study of Thermoplastic Elastomer and Its Industrialization
- Core Invention Points:
- Reviews chemical synthesis type TPE and rubber-plastic blending type TPE.
- Discusses new thermoplastic elastomer products, preparation methods, performance characteristics, and market development.

Potential Breakthroughs in Medical Elastomer Applications
TPE Materials for Specific Applications
Formulated for medical supplies, non-pneumatic tires, and coating or encapsulating other materials like nylon and glass fibers.
Heat-Resistant and High-Temperature TPE Materials
Formulated to exhibit high heat resistance for applications involving high temperatures.
Conductive and Dielectric TPE Materials
Formulated for specific electrical properties like high conductivity or low dielectric constant for electronics and electrical components.
Antibacterial and Functional TPE Materials
Formulated with antibacterial properties or other functional characteristics for healthcare, food processing, and consumer products.
TPE Materials with Improved Mechanical Properties
Engineered for enhanced mechanical properties like high elasticity, wear resistance, and fatigue resistance.
Regulatory Landscape for Medical Elastomers
The regulatory landscape for medical elastomers is complex and governed by various national and international bodies to ensure safety, efficacy, and quality.
United States
- FDA: Oversees medical devices and materials, classifying them based on risk. TPEs used in medical applications are typically Class II or III devices. Manufacturers must comply with premarket approval, 510(k) clearance, GMP, labeling, and post-market surveillance.
European Union
- MDR: Enhances patient safety with stricter requirements for clinical data, post-market surveillance, and traceability.
Other Regions
- Health Canada, TGA (Australia), PMDA (Japan): Have specific regulations for medical devices and materials including TPEs.
Compliance
- Essential for global market entry. Collaboration with regulatory experts, adherence to quality management systems, and continuous monitoring of regulatory changes are key.
Environmental Impact of Medical Elastomer Use
The use of TPE materials in the medical field has environmental impacts across their lifecycle.
Manufacturing Process
- Generates greenhouse gas emissions, consumes significant energy, and potentially releases harmful byproducts.
Usage Phase
- Medical devices made from TPEs may release microplastics or leach harmful substances.
End-of-Life Stage
- TPEs are often difficult to recycle or biodegrade, leading to potential accumulation in landfills or the environment.
Solutions
- Implement cleaner production processes, explore alternative materials, promote circular economy principles through recycling and reuse initiatives.
- Conduct rigorous life cycle assessments and environmental impact studies.
- Collaboration between manufacturers, healthcare providers, and regulatory bodies to develop sustainable practices.
If you want an in-depth research or a technical report, you can always get what you want in Eureka Technical Research. Try now!

