
Overview
The US market currently has 1 approved drug under the name Ocrevus (generic name: Ocrelizumab), which represents a significant therapeutic option for multiple sclerosis treatment. Developed by Genentech, Inc. and Biogen Idec Ltd., Ocrevus was approved in 2017 and has shown strong clinical efficacy in both relapsing and primary progressive forms of multiple sclerosis. The drug operates through a unique mechanism targeting CD20-expressing B-cells, leading to B-cell depletion primarily through antibody-dependent cellular cytolysis and complement-mediated lysis. With patent protection extending to 2028 through a Patent Term Extension (PTE) and a breakthrough therapy designation for primary progressive multiple sclerosis, Ocrevus maintains a strong market position with limited biosimilar competition at present.
Detailed Description
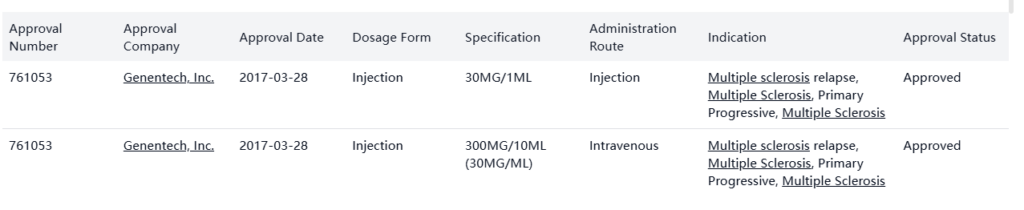
Drug Approval
Ocrevus was developed by Genentech, Inc. and Biogen Idec Ltd., and was officially launched in the USA in 2017.
- Bio-similars
There are 6 bio-similars, for example: Ocrelizumab Biosimilar (Celltrion).
- Special Review
| Organization | Indication | Special Review | Country | Approval Date |
|---|---|---|---|---|
| Genentech, Inc. | Multiple Sclerosis | Fast Track | United States | 2013-03-06 |
| Genentech, Inc. | Multiple Sclerosis, Primary Progressive | Breakthrough Therapy | United States | 2016-02-16 |
Patent Barrier
Registration Patent Analysis
Patent Term Extension (PTE) patents from Genentech, Inc. provide protection until October 2028.
| Patent Number | Simple Legal Status | Application Date | Estimated Expiry | Patent Type | Applicant | Source |
|---|---|---|---|---|---|---|
| US7799900B2 | Active | 2005-06-07 | 2028-10-02 | Sequence | Genentech, Inc. | PTE |
| US20060034835A1 | Active | 2005-06-07 | 2028-10-02 | Sequence | Genentech, Inc. | PTE |
Other Patent Barrier Analysis
Genentech has numerous patents covering various aspects of Ocrelizumab, including new uses, processes, and formulations. Many are international PCT applications that have expired at the designated stage, but the US patents remain active. Key patents include:
| Patent Number | Simple Legal Status | Application Date | Estimated Expiry | Patent Type | Applicant |
|---|---|---|---|---|---|
| US20070212733A1 | Active | 2006-11-21 | 2027-11-08 | New Use, Diagnostic, Analysis and Assay | Genentech, Inc. |
| D3564194-0283-44CC-B584-31F145EF3F05 | Inactive | 2003-12-16 | 2023-12-16 | – | Genentech, Inc. |
Additionally, there are several third-party patents that could impact Ocrelizumab:

Clinical Results
Based on the FDA Label Clinical Insight tool, the following clinical results are significant:
- Nonclinical Toxicology Studies:
- Fertility studies in monkeys showed no adverse effects on reproductive organs at doses 2-10 times higher than the recommended human dose of 600 mg.
- No carcinogenicity studies have been performed, and mutagenicity studies were not conducted as the antibody is not expected to interact directly with DNA.
- Mechanism of Action and Pharmacodynamics:
- Ocrelizumab targets CD20-expressing B-cells, causing B-cell depletion through antibody-dependent cellular cytolysis and complement-mediated lysis.
- B-cell reduction was observed as early as 14 days post-infusion, with a median recovery time of approximately 72 weeks (range: 27–175 weeks).
- Clinical Efficacy in Multiple Sclerosis:
- Relapsing Multiple Sclerosis (RMS): Efficacy was assessed based on relapse rate and disability progression in randomized controlled trials.
- Primary Progressive Multiple Sclerosis (PPMS) Study:
- Randomized, double-blind, placebo-controlled trial with 2:1 randomization to 600 mg ocrelizumab or placebo every 24 weeks.
- Primary endpoint was time to onset of disability progression (confirmed at 12+ weeks).
- Additional outcomes included timed 25-foot walk and percentage change in T2 hyperintense lesion volume on MRI.
- Vaccine Response and Immunogenicity:
- Ocrelizumab attenuated antibody responses to non-live vaccines (tetanus toxoid-containing, pneumococcal, and seasonal influenza).
- In clinical trials, approximately 1% of 1311 treated patients tested positive for anti-drug antibodies (ADAs).
Infringement Cases
Based on the Generic Drug Infringement News tool results, there are no patent infringement incidents involving ocrelizumab reported.
Policy and Regulatory Risk Warning
After a comprehensive search, Ocrevus has Breakthrough Therapy designation for Primary Progressive Multiple Sclerosis in the United States, which provides certain regulatory benefits but does not extend market exclusivity beyond the patent protection period. The drug is protected primarily by patents rather than regulatory exclusivity at this point.
Market Entry Assessment & Recommendations
Based on the comprehensive analysis, the following market assessment and recommendations can be provided:
- Patent Protection: Ocrevus is currently protected by PTE patents until October 2028, providing a strong barrier to generic entry in the US market for the next few years.
- Biosimilar Development Strategy: For companies considering biosimilar development, preparation should begin now to be ready for market entry after 2028. This includes:
- Conducting comprehensive patent landscape analysis to identify potential secondary patent barriers
- Initiating clinical trials well in advance to ensure timely approval after patent expiry
- Developing manufacturing processes that avoid infringement of any valid process patents
- Market Differentiation: For the originator (Genentech/Roche):
- Focus on patient services and support programs to build brand loyalty
- Continue generating real-world evidence on long-term safety and efficacy
- Explore new indications or modified dosing regimens to extend the product lifecycle
- Consider developing next-generation anti-CD20 therapies with improved profiles
- Pricing and Access Strategies:
- For biosimilar developers: Plan competitive pricing strategies to gain market share
- For the originator: Consider value-based contracting with payers to maintain market position
- Both: Develop robust patient assistance programs to ensure continued access
- Regulatory Considerations:
- Monitor any changes in FDA guidance for biosimilar development for complex biologics
- Leverage the FDA’s biosimilar development programs for expedited review where applicable
- Manufacturing Considerations:
- Invest in robust manufacturing processes to ensure consistent quality and supply
- Consider strategic partnerships to optimize manufacturing costs and capabilities
The market for Ocrevus remains strong with limited immediate competition, but strategic planning for post-2028 scenarios should be a priority for both the originator and potential competitors.
For more scientific and detailed information of Ocrevus, try PatSnap Eureka Pharma CI Explorer.
Only takes 4 steps to unlock the detailed result 👇

