
Introduction
When exploring the magnetic properties of materials, understanding paramagnetic vs. diamagnetic vs. ferromagnetic behavior is essential. These terms describe how materials respond to external magnetic fields. Paramagnetic materials, such as aluminum and oxygen, weakly attract magnetic fields due to unpaired electrons. In contrast, diamagnetic materials, including copper and bismuth, exhibit weak repulsion as their electrons are paired, canceling magnetic moments. Ferromagnetic materials like iron, cobalt, and nickel strongly attract and retain magnetism, making them essential in permanent magnets and electronic devices. Comparing paramagnetic vs. diamagnetic vs. ferromagnetic properties reveals the diverse ways materials interact with magnetic fields, influencing applications across industries.
What Are Diamagnetic Materials?
Diamagnetic materials lack permanent magnetic moments because all their electrons are paired, resulting in no net atomic magnetism. When exposed to an external magnetic field, these materials generate a weak, opposing magnetic field, creating a repulsive effect. This behavior stems from subtle changes in the orbital motion of electrons influenced by the applied field.
Key Properties of Diamagnetic Materials
- Magnetic Susceptibility: Diamagnetic materials have negative magnetic susceptibility (χ), indicating repulsion by magnetic fields. This susceptibility is weak, often less than -0.5 or even -0.75.
- Behavior in Magnetic Fields: Unlike paramagnetic or ferromagnetic materials, diamagnetic materials show no residual magnetization once the external field is removed. Their negative response to magnetic fields remains consistent regardless of temperature.
What Are Paramagnetic Materials?
Paramagnetic materials exhibit weak attraction to external magnetic fields but do not retain magnetization once the field is removed. This behavior occurs because the magnetic moments of their atoms or ions are randomly oriented due to thermal motion, resulting in no net magnetization without an applied field.
Key Properties of Paramagnetic Materials
- Magnetic Susceptibility: Paramagnetic materials have positive magnetic susceptibility, typically much less than one. Their magnetic moments align weakly with the applied field, creating a net magnetization that is proportional to the field strength. However, this magnetization is significantly weaker than that of ferromagnetic materials.
- Temperature Dependence: The magnetic behavior of paramagnetic materials is strongly temperature-dependent. According to Curie’s Law, their magnetic susceptibility inversely correlates with temperature, expressed as χ = C/T. As the temperature rises, thermal agitation disrupts the alignment of magnetic moments, decreasing the material’s overall magnetization.
What Are Ferromagnetic Materials?
Ferromagnetic materials exhibit strong magnetism, becoming highly magnetized when exposed to an external field and retaining magnetization after the field is removed. This behavior arises from the alignment of magnetic moments within the material. Examples include iron, cobalt, nickel, and their alloys.
Properties of Ferromagnetic Materials
- Magnetization and Hysteresis
Ferromagnetic materials display hysteresis, where their magnetization increases to a saturation point under an external magnetic field. Even after the field is removed, residual magnetization remains due to the alignment of magnetic domains. This characteristic makes these materials essential for applications like permanent magnets. - Curie Temperature
The Curie temperature is the threshold above which ferromagnetic materials lose their magnetism and become paramagnetic. This value varies among materials—for example, iron’s Curie temperature is 770°C, while cobalt’s is 1,115°C. - Magnetic Domains
Ferromagnetic materials consist of magnetic domains, regions where atomic magnetic moments align in the same direction. Domain walls, or boundaries between these regions, shift under an applied field, creating the material’s magnetic properties. - Saturation Magnetization and Coercivity
Saturation magnetization refers to the maximum magnetization achievable under an external field. Coercivity measures a material’s resistance to demagnetization. High-coercivity materials are ideal for permanent magnets, while low-coercivity materials excel in soft magnetic applications like transformers.
Paramagnetic vs. Diamagnetic vs. Ferromagnetic: Key Defferences
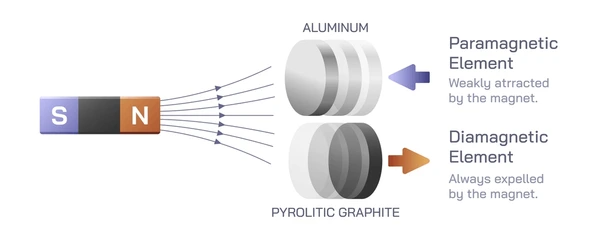
Fundamental Properties and Behaviors
- Paramagnetic Materials
Paramagnetic materials have a small positive magnetic susceptibility, meaning they are weakly attracted by an external magnetic field. Their susceptibility decreases as temperature rises, following the Curie-Weiss law. These materials contain unpaired electrons that align with the magnetic field, creating a net magnetic moment. However, they lose magnetization once the field is removed due to thermal agitation. - Diamagnetic Materials
Diamagnetic materials exhibit a negative magnetic susceptibility, causing them to be weakly repelled by magnetic fields. This property is independent of temperature and arises from quantum mechanics. Their electrons are all paired, resulting in no net magnetic moment. When exposed to a magnetic field, they induce an opposing field, creating a weak repulsion. - Ferromagnetic Materials
Ferromagnetic materials have extremely high positive magnetic susceptibility, often thousands of times greater than paramagnetic materials. This decreases with temperature and vanishes above the Curie point. Unpaired electrons align due to strong exchange interactions, forming magnetic domains. These materials retain magnetization after the field is removed, making them ideal for permanent magnets.
Internal Structures
- Paramagnetic Materials
Paramagnetic materials, like aluminum and platinum, have unpaired electrons that create a net magnetic moment. These moments align temporarily with an external field, generating weak and reversible magnetization. - Diamagnetic Materials
Materials like graphite and copper are diamagnetic, with paired electrons causing no permanent magnetic moment. They oppose applied magnetic fields, resulting in weak repulsion, though stronger magnetic effects in mixed materials can overshadow this behavior. - Ferromagnetic Materials
Ferromagnetic materials, such as iron, cobalt, and nickel, exhibit strong exchange interactions among unpaired electrons, forming aligned magnetic domains. This structure creates their remarkable ability to maintain strong magnetization and exhibit hysteresis.
Practical Applications
- Paramagnetic Materials
Paramagnetic materials are essential in MRI contrast agents, specific sensors, and research on magnetic properties. Their weak magnetization limits broader applications. - Diamagnetic Materials
Diamagnetic materials find use in magnetic levitation, shielding, and non-magnetic electronic components. Their weak repulsion restricts use in high-interaction magnetic applications. - Ferromagnetic Materials
Ferromagnetic materials dominate applications like permanent magnets, transformers, and magnetic storage media. While highly effective, they lose magnetization above the Curie temperature and can experience hysteresis losses.
How to Determine the Magnetic Properties of a Material
Magnetic Susceptibility Measurement
Volume Magnetic Susceptibility (χ): This property determines whether a material is diamagnetic, paramagnetic, or ferromagnetic. Advanced instruments typically measure χ accurately, but cost-effective alternatives exist. For instance, using a balance and neodymium magnets allows affordable measurement. By optimizing the sample’s size, shape, and position, uncertainties in experimental results are minimized.
Hysteresis Curve Analysis
Hysteresis Curves: These curves reveal crucial magnetic properties like remanence magnetization, coercivity, and saturation magnetization. Devices such as vibrating sample magnetometers (VSM), SQUIDs, and MOKE systems are commonly employed. These tools effectively measure how materials respond to magnetic fields under varying conditions.
FAQ
How do paramagnetic and diamagnetic materials respond to external magnetic fields?
- Diamagnetic materials develop an induced magnetic field in the opposite direction of the applied field, causing a weak repulsion.
- Paramagnetic materials align their unpaired electron spins with the external field, resulting in a weak attraction.
Can ferromagnetic materials become permanently magnetized?
- Yes, ferromagnetic materials can become permanently magnetized because their magnetic domains can remain aligned even after the external magnetic field is removed.
What are some examples of diamagnetic, paramagnetic, and ferromagnetic materials?
- Diamagnetic: Copper, silver, gold.
- Paramagnetic: Aluminum, platinum, manganese.
- Ferromagnetic: Iron, cobalt, nickel.
To get detailed scientific explanations of Paramagnetic vs. Diamagnetic vs. Ferromagnetic, try Patsnap Eureka.

