
In organic chemistry, understanding nucleophilic substitution reactions is essential for predicting how molecules behave. Two primary types of substitution reactions—SN1 vs. SN2—follow different mechanisms and are influenced by various factors like solvent, structure, and nucleophile strength. This article breaks down the differences between SN1 and SN2 mechanisms, covering their rate laws, reactivity trends, solvent preferences, and key examples to help you master these foundational reactions.
What Are SN1 and SN2 Reactions?
SN1 or SN2? Eureka Technical Q&A breaks down these classic substitution reactions—one step or two, fast or slow—so you can master the mechanism and pick the right path in organic chemistry.
SN1 Reaction
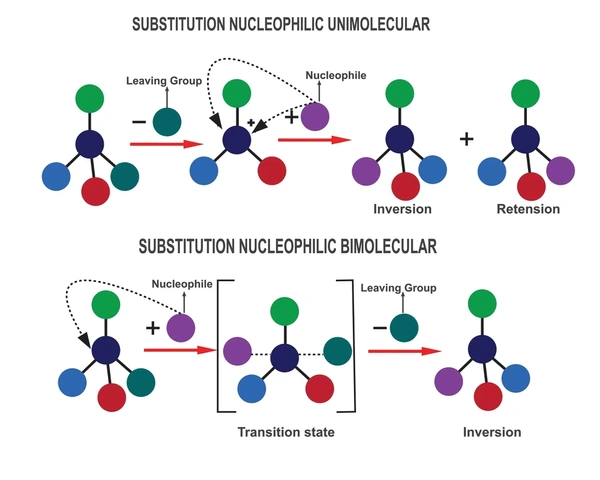
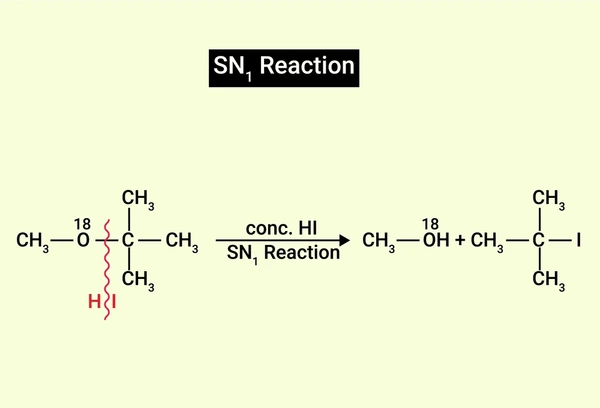
- Mechanism: The SN1 reaction follows a two-step, unimolecular nucleophilic substitution process. First, the substrate dissociates into a carbocation and a leaving group. Next, the nucleophile attacks the carbocation from either side. This results in a mix of products with inverted or retained configuration.
- Characteristics: This reaction typically occurs with tertiary or secondary alkyl halides. It proceeds best in polar protic solvents that stabilize the carbocation intermediate. Moreover, the SN1 reaction is first-order and depends only on the substrate concentration.
- Products: The reaction yields both inverted and retained configurations at the reactive center. Consequently, it often produces a racemic mixture of enantiomers.
SN2 Reaction
- Mechanism: The SN2 reaction is a bimolecular nucleophilic substitution mechanism. It proceeds in one step with no intermediate formation. The nucleophile attacks the substrate from the backside of the leaving group. The new bond forms as the leaving group departs simultaneously. This leads to complete inversion of configuration at the reactive carbon center. This results in an inversion of configuration at the carbon center.
- Characteristics: This mechanism is common with primary and secondary alkyl halides and is favored by strong nucleophiles and poor leaving groups. It is a second-order reaction, depending on the concentrations of both the substrate and the nucleophile.
- Products: The reaction consistently leads to inversion of configuration, resulting in a single product with a fixed stereochemistry.
SN1 vs. SN2: Rate Laws
| Reaction Type | Rate Law | Depends On |
|---|---|---|
| SN1 | Rate = k [substrate] | Only substrate |
| SN2 | Rate = k [substrate][nucleophile] | Substrate and nucleophile |
SN2 Reaction: This is a bimolecular reaction, meaning it involves two molecules: the nucleophile and the alkyl halide. The rate law for an SN2 reaction is typically second-order, with the rate depending on the concentration of both the nucleophile and the alkyl halide. The reaction proceeds in a single step with a transition state where the nucleophile attacks the backside of the alkyl halide, resulting in the inversion of configuration at the carbon atom undergoing substitution.
SN1 Reaction: This is a unimolecular reaction, involving only one molecule, the alkyl halide. The rate-determining step is the formation of a carbocation intermediate from the alkyl halide. The rate law for an SN1 reaction is first-order, depending only on the concentration of the alkyl halide. The reaction proceeds through a two-step mechanism: first, the departure of the leaving group to form a carbocation, followed by the attack of the nucleophile on the carbocation.
Reactivity of Substrates
| Substrate Type | SN1 Reactivity | SN2 Reactivity |
|---|---|---|
| Methyl | Not reactive | Very fast |
| Primary (1°) | Slow | Fast |
| Secondary (2°) | Moderate | Moderate |
| Tertiary (3°) | Fast | Very slow or not at all |
SN1 is favored by stable carbocations, so tertiary substrates react faster. SN2 is favored by less hindered substrates, such as methyl or primary carbon centers.
Nucleophile Strength
- SN1: Nucleophile strength is less important
- SN2: Strong nucleophiles are essential
Examples of strong nucleophiles: OH⁻, CN⁻, I⁻, and SH⁻
Weak nucleophiles (like H₂O or ROH) still work in SN1 due to carbocation formation.
Solvent Effects
- SN2 Reactions: Polar aprotic solvents, such as acetonitrile or dimethyl sulfoxide, are preferred for SN2 reactions. These solvents stabilize the negative charge on the nucleophile by solvating it, lowering the energy of the transition state and increasing the reaction rate.
- SN1 Reactions: Polar protic solvents, such as water or ethanol, are often used in SN1 reactions. These solvents can stabilize the carbocation intermediate through hydrogen bonding, making the reaction more favorable.
| Solvent Type | SN1 Reactions | SN2 Reactions |
|---|---|---|
| Polar protic solvents | Favor SN1 (stabilize ions) | Inhibit SN2 (solvate nucleophile) |
| Polar aprotic solvents | Inhibit SN1 | Favor SN2 (nucleophile remains reactive) |
Examples:
- Protic: Water, methanol, ethanol
- Aprotic: Acetone, DMSO, DMF
Stereochemistry
- SN1: Leads to racemic mixture (loss of stereochemistry)
- SN2: Causes inversion of configuration at the reactive center (backside attack)
Common Examples
SN1 Example
Tert-butyl bromide + H₂O → tert-butyl alcohol + HBr
(Carbon forms a stable tertiary carbocation)
SN2 Example
Methyl bromide + NaOH → Methanol + NaBr
(One-step backside attack with strong nucleophile)
Summary Table: SN1 vs. SN2
- Order: SN1 is unimolecular (first-order), while SN2 is bimolecular (second-order).
- Steps: SN1 involves a two-step process with a carbocation intermediate, whereas SN2 is a single-step process.
- Stereochemistry: SN1 can result in a racemic mixture due to the carbocation’s ability to be attacked from either side, while SN2 consistently causes inversion of configuration.
- Conditions: SN1 is favored in protic solvents and with tertiary substrates, whereas SN2 is favored in aprotic solvents and with primary or secondary substrates.
| Feature | SN1 | SN2 |
|---|---|---|
| Mechanism | Two-step (carbocation intermediate) | One-step (concerted) |
| Rate Law | Rate = k [substrate] | Rate = k [substrate][nucleophile] |
| Substrate Preference | Tertiary > Secondary > Primary | Methyl > Primary > Secondary |
| Nucleophile Role | Weak nucleophile acceptable | Strong nucleophile required |
| Solvent Preference | Polar protic | Polar aprotic |
| Stereochemistry | Racemization | Inversion of configuration |
FAQs
Tertiary carbocations are more stable due to alkyl group stabilization, making carbocation formation easier in SN1 reactions.
No. Tertiary substrates are too sterically hindered, preventing backside attack.
It depends on conditions. SN2 is usually faster with strong nucleophiles and less hindered substrates, while SN1 is faster with stable carbocations in polar protic solvents.
Polar aprotic solvents like acetone or DMSO enhance nucleophilicity, making SN2 reactions proceed more efficiently.
Typically yes, due to the planar nature of the carbocation intermediate. However, slight stereoselectivity can occur in some cases.
Conclusion
Understanding the differences between SN1 and SN2 reactions is essential for predicting reaction pathways in organic chemistry. While both achieve the same substitution goal, they do so through different mechanisms influenced by substrate structure, nucleophile strength, solvent type, and stereochemistry. Knowing these distinctions allows chemists to choose the right conditions for desired outcomes in synthesis and reaction design.
To get detailed scientific explanations of SN1 vs. SN2, try Patsnap Eureka.


