
Sulfamic acid (HSO₃NH₂), also known as amidosulfonic acid, is a versatile compound used in a variety of industrial and laboratory applications. It is a strong acid and a key ingredient in many cleaning and descaling agents. This article will explore the properties, uses, safety considerations, and common questions regarding sulfamic acid.
What is Sulfamic Acid?
Sulfamic acid is an inorganic acid that consists of a sulfur atom bonded to an amine group and a sulfonic acid group. Its chemical formula is HSO₃NH₂. It is typically available as a white, crystalline solid that is highly soluble in water. HSO₃NH₂ is a strong acid, often used as a safer alternative to hydrochloric acid in cleaning applications.
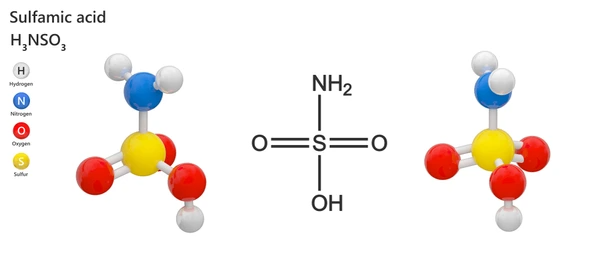
Key Properties of Sulfamic Acid
- Chemical Formula: HSO₃NH₂
- Appearance: White, crystalline solid
- Solubility: Soluble in water
- Acidity: Strong acid
- pH: Typically acidic when dissolved in water
- Melting Point: 205°C
Uses of Sulfamic Acid
- Descaling and Cleaning Agents: Industries commonly use HSO₃NH₂ in descaling formulations to remove lime scale, rust, and mineral deposits from boilers, pipes, and water systems. They often prefer it over hydrochloric acid for its safer handling and less aggressive nature.
- Water Treatment In water treatment plants, HSO₃NH₂ is used for neutralizing alkalis and as a cleaning agent for reverse osmosis membranes. It helps in breaking down mineral build-ups that can obstruct the filtration process.
- Chemical Synthesis HSO₃NH₂ is used in the synthesis of various chemicals, including sulfonamides (a group of antibiotics), herbicides, and pesticides. Its high reactivity with organic compounds makes it valuable in the production of sulfonic derivatives.
- pH Control in Industries HSO₃NH₂ can act as a pH adjuster in certain processes, particularly where precision control of the pH level is required.
- Manufacture of Cleaning Products It is an important ingredient in cleaning products, such as toilet bowl cleaners, where its ability to break down mineral deposits proves to be effective.
💡 Sulfamic acid plays a crucial role in industries like cleaning, water treatment, and chemical manufacturing. Want to learn more about its applications and benefits? Eureka Technical Q&A provides expert insights into how sulfamic acid is used for descaling, pH regulation, and industrial processing, ensuring you maximize its potential safely and effectively.
Safety Considerations When Handling Sulfamic Acid
- Skin Contact: Direct contact with HSO₃NH₂ can cause skin irritation or burns. Wear gloves and protective clothing to minimize contact.
- Eye Contact: HSO₃NH₂ can cause severe irritation and damage to the eyes. In case of contact, immediately flush the eyes with water for at least 15 minutes and seek medical attention.
- Inhalation: Breathing in vapors or dust may irritate the respiratory system. Work in well-ventilated areas or use appropriate respiratory protection.
- Ingestion: If ingested, HSO₃NH₂ can cause serious internal damage. Seek immediate medical attention if swallowing occurs.
- Storage: Store HSO₃NH₂ in cool, dry places, away from incompatible materials like strong bases and oxidizing agents. Keep it in airtight containers to prevent moisture absorption.
Environmental Impact of Sulfamic Acid
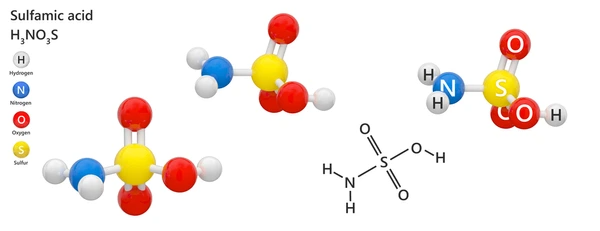
HSO₃NH₂ is generally considered less harmful to the environment than some other acids, such as hydrochloric acid. However, large spills can lead to soil acidification and potential harm to aquatic ecosystems. Proper disposal and treatment of HSO₃NH₂ waste are essential to minimize environmental impact.
Sulfamic Acid vs. Hydrochloric Acid: Key Differences
| Property | Sulfamic Acid | Hydrochloric Acid |
|---|---|---|
| Chemical Composition | Amidosulfonic acid (HSO₃NH₂) | Hydrogen chloride (HCl) |
| Acidity | Strong, but less aggressive | Highly aggressive, stronger |
| Uses | Descaling, water treatment, synthesis | Cleaning, pH control, industrial applications |
| Corrosivity | Less corrosive than HCl | Highly corrosive |
| Safety | Safer, less toxic | Requires more caution |
Conclusion
Sulfamic acid is a versatile, effective, and safer alternative to stronger acids for cleaning, descaling, water treatment, and chemical synthesis. Its controlled reactivity makes it ideal for various applications while requiring proper handling and disposal. With safe use and environmental precautions, HSO₃NH₂ remains a valuable chemical in industrial and household settings.
FAQs
1. What is the difference between sulfamic acid and sulfuric acid?
HSO₃NH₂ is a sulfonic acid, while sulfuric acid is a mineral acid. HSO₃NH₂ is generally safer to handle, less aggressive, and primarily used for cleaning and descaling, whereas sulfuric acid is a stronger and more corrosive acid used in a broader range of industrial applications.
2. Is sulfamic acid safe for home use?
Use HSO₃NH₂ for cleaning tasks like descaling toilets and boilers in residential settings, but take precautions. Wear gloves and eye protection, and keep the area well-ventilated.Always follow the manufacturer’s safety guidelines when using cleaning products containing HSO₃NH₂.
3. Can sulfamic acid be used to clean stainless steel?
Yes, use HSO₃NH₂ to clean stainless steel surfaces, especially for removing lime scale or mineral deposits. However, avoid prolonged exposure or improper concentration to prevent surface damage. Always follow recommended guidelines.
4. How do you neutralize sulfamic acid?
HSO₃NH₂ can be neutralized with sodium bicarbonate (baking soda) or lime. Always add neutralizing agents gradually to avoid excessive heat release or splashing.
5. Can sulfamic acid be used to clean swimming pools?
While HSO₃NH₂ effectively removes calcium deposits and mineral stains, users should carefully dilute it before applying it in swimming pools. The use of such acids may alter the pH levels of pool water, requiring subsequent adjustments.
To get detailed scientific explanations of Sulfamic Acid, try Patsnap Eureka.


