
Valence Shell Electron Pair Repulsion (VSEPR) theory helps predict the three-dimensional shape of molecules by considering how electron pairs around a central atom repel each other. This article will break down the basics of VSEPR theory, key molecular shapes, bond angles, and how electron pair geometry differs from molecular geometry.
Why do molecules have different shapes? Eureka Q&A breaks down VSEPR Theory—predicting molecular geometry, bond angles, and electron pair arrangements with clarity. Perfect for students, chemists, and science lovers!
What Is VSEPR Theory?
VSEPR theory stands for Valence Shell Electron Pair Repulsion theory. It is based on the idea that electron pairs (bonding and lone pairs) around a central atom will arrange themselves as far apart as possible to minimize repulsion. This spatial arrangement determines the shape and geometry of the molecule.
Basic Principles: VSEPR theory is based on the idea that electron pairs, including both bonding and lone pairs, repel each other. This repulsion influences the molecular geometry, causing the molecule to adopt a shape that minimizes this repulsion.
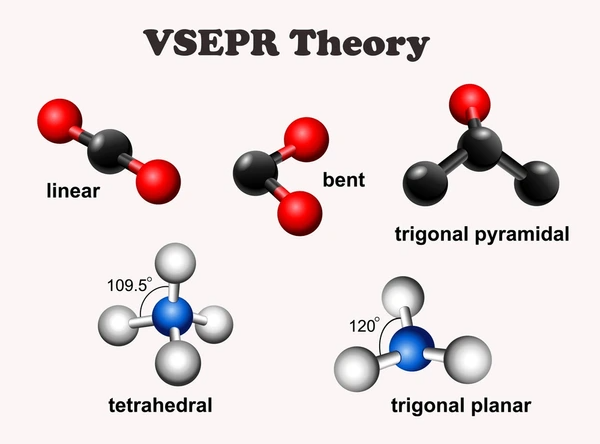
Key Concepts of VSEPR Theory
- Electron domains: Regions of electron density (lone pairs or bonds).
- Bonding pairs: Electrons shared between atoms (single, double, or triple bonds count as one domain).
- Lone pairs: Non-bonding electrons localized on a single atom.
- Molecular geometry: Shape formed by atoms only.
- Electron pair geometry: Shape formed by all electron domains around the central atom.
VSEPR Notation (AXE Method)
- A = Central atom
- X = Number of bonded atoms
- E = Number of lone electron pairs on the central atom
Example: AX₂E₂ refers to a molecule with 2 bonded atoms and 2 lone pairs on the central atom (e.g., H₂O).
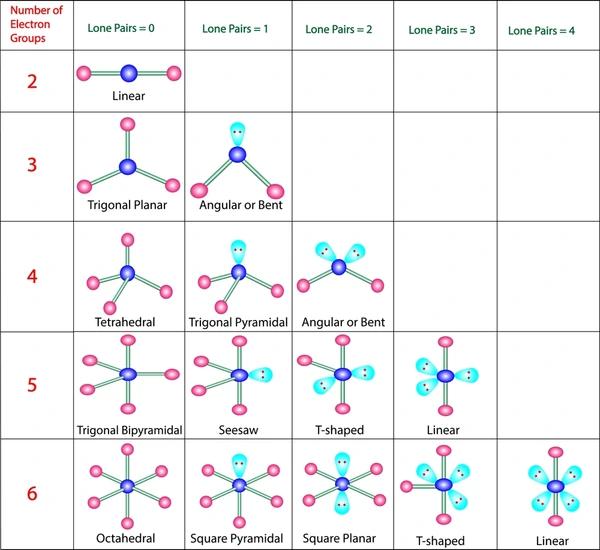
Predicting Shapes
The theory predicts specific shapes for molecules based on the number of electron pairs around the central atom. For instance:
- Linear shape with 180° angles occurs when there are two electron pairs (bonding pairs only).
- Trigonal planar shape with 120° angles occurs when there are three electron pairs (bonding pairs only).
- Tetrahedral shape with 109.5° angles occurs when there are four electron pairs (bonding pairs only).
- Trigonal bipyramidal shape occurs when there are five electron pairs (bonding pairs only).
- Octahedral shape occurs when there are six electron pairs (bonding pairs only) .
Electron Pair Geometry vs. Molecular Geometry
- Electron Pair Geometry: This refers to the arrangement of electron pairs around a central atom in a molecule. It includes both bonding and lone pairs of electrons. The electron pair geometry is primarily determined by the number of electron pairs, whether they are bonding pairs (between atoms) or lone pairs (non-bonding pairs on a single atom). The VSEPR (Valence Shell Electron Pair Repulsion) theory is often used to predict the electron pair geometry, as it considers the repulsion between electron pairs to find the most stable arrangement.
- Molecular Geometry: This describes the actual three-dimensional shape of a molecule based on how the atoms are connected by covalent bonds. While molecular geometry considers the positions of atoms in space, electron pair geometry looks at the arrangement of electron pairs, which can include both bonding and lone pairs. Therefore, the molecular geometry can differ from the electron pair geometry, especially when there are lone pairs present on the central atom, as lone pairs occupy more space than bonding pairs.
| Electron Domains | Electron Geometry | Molecular Geometry (with Lone Pairs) |
|---|---|---|
| 2 | Linear | Linear |
| 3 | Trigonal Planar | Bent (1 lone pair) |
| 4 | Tetrahedral | Trigonal pyramidal / Bent |
| 5 | Trigonal Bipyramidal | Seesaw / T-Shaped / Linear |
| 6 | Octahedral | Square pyramidal / Square planar |
VSEPR Shapes and Bond Angles
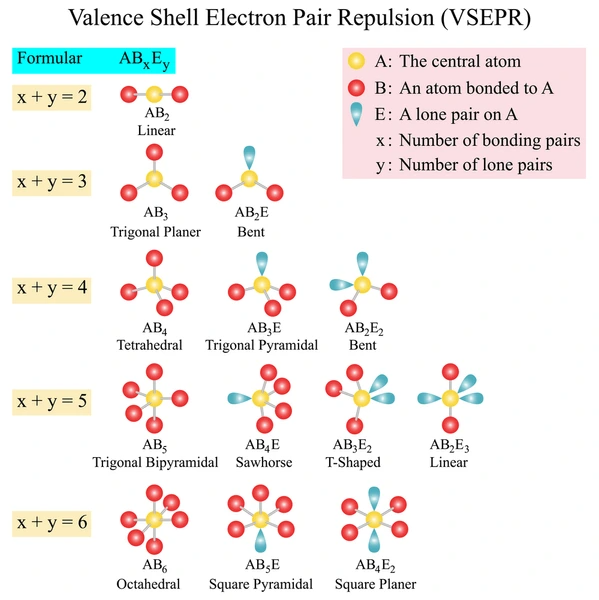
Here’s a breakdown of common molecular geometries and their ideal bond angles:
1. Linear (AX₂)
- Bond Angle: 180°
- Example: CO₂
2. Trigonal Planar (AX₃)
- Bond Angle: 120°
- Example: BF₃
3. Bent (AX₂E or AX₂E₂)
- Bond Angles:
- ~117° (1 lone pair, e.g., SO₂)
- ~104.5° (2 lone pairs, e.g., H₂O)
4. Tetrahedral (AX₄)
- Bond Angle: 109.5°
- Example: CH₄
5. Trigonal Pyramidal (AX₃E)
- Bond Angle: ~107°
- Example: NH₃
6. Trigonal Bipyramidal (AX₅)
- Bond Angles: 90° and 120°
- Example: PCl₅
7. Seesaw (AX₄E)
- Bond Angles: ~90°, ~120°
- Example: SF₄
8. T-Shaped (AX₃E₂)
- Bond Angles: ~90°
- Example: ClF₃
9. Octahedral (AX₆)
- Bond Angle: 90°
- Example: SF₆
10. Square Pyramidal (AX₅E)
- Bond Angles: ~90°
- Example: BrF₅
11. Square Planar (AX₄E₂)
- Bond Angles: 90°
- Example: XeF₄
How Lone Pairs Affect Molecular Shape
Lone pairs occupy more space than bonding pairs, causing:
- Bond angle compression
- Distorted geometries
- Molecular shapes different from electron pair geometries
Example:
In H₂O (AX₂E₂), the two lone pairs push the hydrogen atoms closer together, reducing the bond angle to ~104.5°.
Steps to Predict Molecular Geometry Using VSEPR
- Draw the Lewis structure of the molecule.
- Count electron domains (bonding + lone pairs) around the central atom.
- Determine electron geometry based on total domains.
- Determine molecular geometry by considering lone pair influence.
- Predict bond angles and shape.
Real-Life Applications of VSEPR Theory
- Drug design: Predicts 3D molecular shape for receptor binding.
- Environmental chemistry: Determines geometry of pollutants.
- Material science: Helps design molecular structures in polymers and crystals.
Summary Table: VSEPR Geometries
| VSEPR Formula | Electron Geometry | Molecular Shape | Bond Angles | Example |
|---|---|---|---|---|
| AX₂ | Linear | Linear | 180° | CO₂ |
| AX₃ | Trigonal Planar | Trigonal Planar | 120° | BF₃ |
| AX₂E | Trigonal Planar | Bent | ~117° | SO₂ |
| AX₄ | Tetrahedral | Tetrahedral | 109.5° | CH₄ |
| AX₃E | Tetrahedral | Trigonal Pyramidal | ~107° | NH₃ |
| AX₂E₂ | Tetrahedral | Bent | ~104.5° | H₂O |
| AX₅ | Trigonal Bipyramidal | Trigonal Bipyramidal | 90°, 120° | PCl₅ |
| AX₄E | Trigonal Bipyramidal | Seesaw | ~90°, 120° | SF₄ |
| AX₃E₂ | Trigonal Bipyramidal | T-Shaped | ~90° | ClF₃ |
| AX₆ | Octahedral | Octahedral | 90° | SF₆ |
| AX₅E | Octahedral | Square Pyramidal | ~90° | BrF₅ |
| AX₄E₂ | Octahedral | Square Planar | 90° | XeF₄ |
Conclusion
VSEPR theory provides a simple yet powerful way to predict molecular geometry based on the repulsion between electron pairs. By understanding electron domain arrangements and applying AXE notation, you can determine the shape, bond angles, and 3D structure of molecules with confidence. Whether you’re solving textbook problems or modeling real-life molecules, VSEPR theory is a foundational tool in your chemistry toolkit.
FAQs
Lone pairs exert greater repulsive forces, pushing bonded atoms closer together and compressing angles.
A double (or triple) bond counts as one electron domain in VSEPR theory.
Electron geometry considers all electron regions, while molecular geometry includes only the bonded atoms.
It can help, as molecular shape affects polarity, but full polarity prediction requires considering electronegativity and dipole vectors.
It’s useful for small molecules and local geometry in large molecules but doesn’t predict the full shape of complex biomolecules.
To get detailed scientific explanations of VSEPR theory, try Patsnap Eureka.


