
Dipole-dipole forces are a type of intermolecular force that occurs between molecules possessing permanent dipoles. These forces arise from the electrostatic attraction between the positive end of one polar molecule and the negative end of another. Dipole-dipole forces play a crucial role in determining the physical properties of substances, including their boiling points, melting points, solubility, and molecular behavior in different environments.
This article explains the concept of dipole-dipole forces, how they work, and why they are important in chemistry, physics, and real-world applications.
What Are Dipole-Dipole Forces in Simple Terms?
Dipole-dipole forces are attractive interactions between polar molecules — molecules that have regions of partial positive and partial negative charge due to differences in electronegativity between bonded atoms.
When two polar molecules approach each other, their oppositely charged regions attract, creating a dipole-dipole interaction. The strength of this attraction depends on:
- The magnitude of the molecular dipole moments
- The distance between the molecules
- The relative orientation of the dipoles
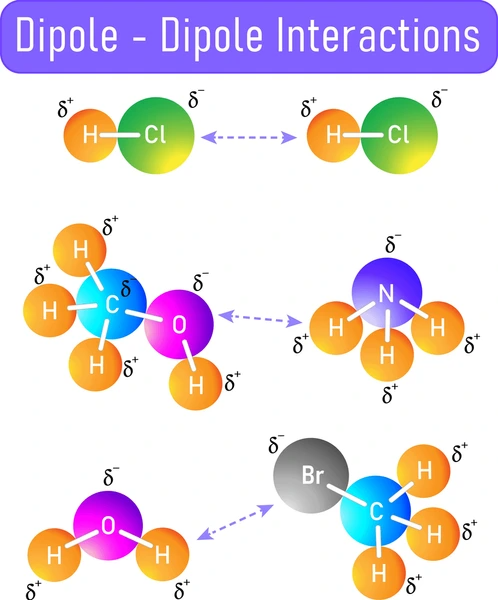
How Do Dipole-Dipole Forces Work?
Dipole-dipole forces operate based on the principle of opposite charges attracting each other. In a polar molecule:
- The more electronegative atom pulls electrons closer, creating a partial negative charge (δ-).
- The less electronegative atom has a partial positive charge (δ+).
When polar molecules are near one another, the positive pole of one molecule aligns with the negative pole of another, leading to an attractive force between them.
Example:
→ In hydrogen chloride (HCl), chlorine is more electronegative than hydrogen, so HCl has a dipole moment.
When HCl molecules come close together, the δ+ hydrogen of one molecule attracts the δ- chlorine of another, forming dipole-dipole forces.
Key Characteristics of Dipole-Dipole Forces
- Occur only between polar molecules
- Stronger than London dispersion forces but weaker than hydrogen bonds
- Strength increases with larger dipole moments
- Affect the boiling and melting points of substances
- Operate over short distances
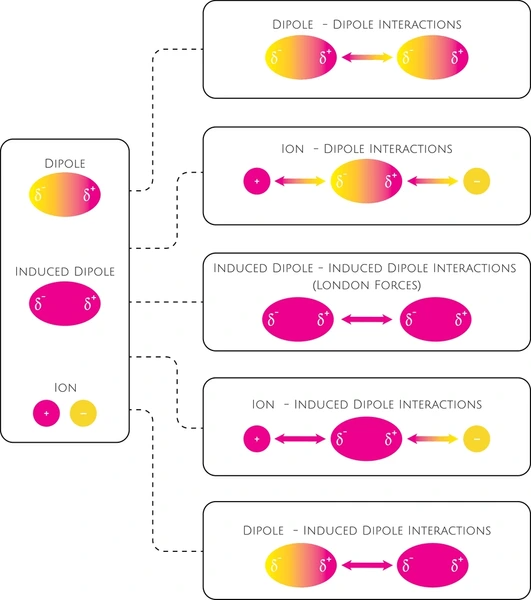
Dipole-Dipole Forces vs. Other Intermolecular Forces
Dipole-dipole forces occur between polar molecules with permanent dipoles. They are stronger than London dispersion forces but weaker than hydrogen and ion-dipole interactions.
- Hydrogen Bonds: A stronger type of dipole-dipole force involving hydrogen and electronegative atoms (e.g., O, N, F); key in water, DNA, and proteins.
- Ion-Dipole Forces: Strongest among these types; form between ions and polar molecules, often in salt solutions.
- London Dispersion Forces: Weak, temporary dipole attractions found in all molecules; strength increases with molecular size.
- Hydrophobic Interactions: Occur between non-polar molecules in water; vital in protein folding and membrane formation.
Each force influences molecular properties such as boiling point, solubility, and biological function.
| Force Type | Occurs Between | Relative Strength | Example |
|---|---|---|---|
| Dipole-Dipole Forces | Polar molecules with permanent dipoles | Moderate | HCl, SO₂, CH₂Cl₂ |
| London Dispersion Forces | All molecules, especially non-polar | Weakest | N₂, CO₂ |
| Hydrogen Bonds | Polar molecules with H bonded to N, O, or F | Strongest intermolecular force | H₂O, NH₃, HF |
Examples of Dipole-Dipole Forces in Molecules
1. Hydrogen Chloride (HCl)
- Polar molecule due to electronegative chlorine
- Dipole-dipole forces align H (δ+) of one molecule with Cl (δ-) of another.
2. Sulfur Dioxide (SO₂)
- Bent shape with net dipole moment
- Molecules attract each other through dipole-dipole interactions.
3. Acetone (C₃H₆O)
- Polar carbonyl group (C=O) creates dipoles
- Strong dipole-dipole attractions influence acetone’s boiling point.
Real-World Importance of Dipole-Dipole Forces
Dipole-dipole forces influence several physical and chemical properties:
1. Boiling and Melting Points
Substances with strong dipole-dipole forces require more energy to separate molecules, resulting in higher boiling and melting points compared to non-polar substances of similar size.
2. Solubility
Polar molecules with dipole-dipole forces dissolve better in polar solvents (like water) due to “like dissolves like” behavior.
3. Molecular Organization
Dipole-dipole interactions affect the arrangement of molecules in liquids and solids, impacting viscosity, surface tension, and material properties.
4. Biological Systems
Dipole-dipole forces are essential in protein folding, DNA structure, and molecular recognition in biochemical processes.
Limitations and Range of Dipole-Dipole Forces
- Operate effectively only over short distances (nanometers or less).
- Weaken rapidly as distance between molecules increases.
- Can be overwhelmed by stronger forces like hydrogen bonding or ionic attractions.
Relation to Hydrogen Bonding
Hydrogen bonding is a special case of dipole-dipole interaction where hydrogen is covalently bonded to highly electronegative atoms like nitrogen, oxygen, or fluorine. Hydrogen bonds are much stronger than typical dipole-dipole forces due to the highly polarized nature of the H-X bond.
Conclusion
Dipole-dipole forces are essential intermolecular forces that govern the behavior of polar molecules. By attracting opposite charges within different molecules, these forces influence boiling points, solubility, and molecular interactions in chemistry and biology.
While not as strong as hydrogen bonds or ionic forces, dipole-dipole interactions are still fundamental to understanding molecular properties, chemical reactions, and the design of materials with desired physical characteristics.
FAQs
Molecules must have a permanent dipole — meaning they are polar with uneven charge distribution.
Yes, dipole-dipole forces are generally stronger because they involve permanent charges rather than temporary ones.
No. Non-polar molecules interact mainly through London dispersion forces.
Yes, but it’s a special, much stronger version involving hydrogen bonded to N, O, or F atoms.
Molecules with strong dipole-dipole attractions need more energy (heat) to separate, leading to higher boiling points.
To get detailed scientific explanations of Dipole-Dipole Force, try Patsnap Eureka.


