
In organic chemistry, chirality plays a critical role in molecular structure and reactivity. But not all molecules with chiral centers behave as chiral compounds. Enter the meso compound—a unique case where optical activity is canceled by internal symmetry. Though it may contain stereocenters, a meso compound is achiral overall. In this article, you’ll learn what a meso compound is, how to identify it, and why it matters, with clear structural examples and tips to spot them easily.
Struggling to understand meso compounds—those molecules with chiral centers that don’t rotate light? Eureka Q&A breaks down their definition, structure, and real examples in seconds, helping you master organic chemistry with clarity.
What Is a Meso Compound?
A meso compound is an achiral compound that contains two or more chiral centers, yet has a plane of symmetry that makes it optically inactive.
Key Characteristics:
- Has stereocenters (usually at least two)
- Contains a mirror plane or center of symmetry
- Superimposable on its mirror image
- Does not rotate plane-polarized light
Even though it looks like it should be chiral, its symmetry causes the optical effects to cancel out.
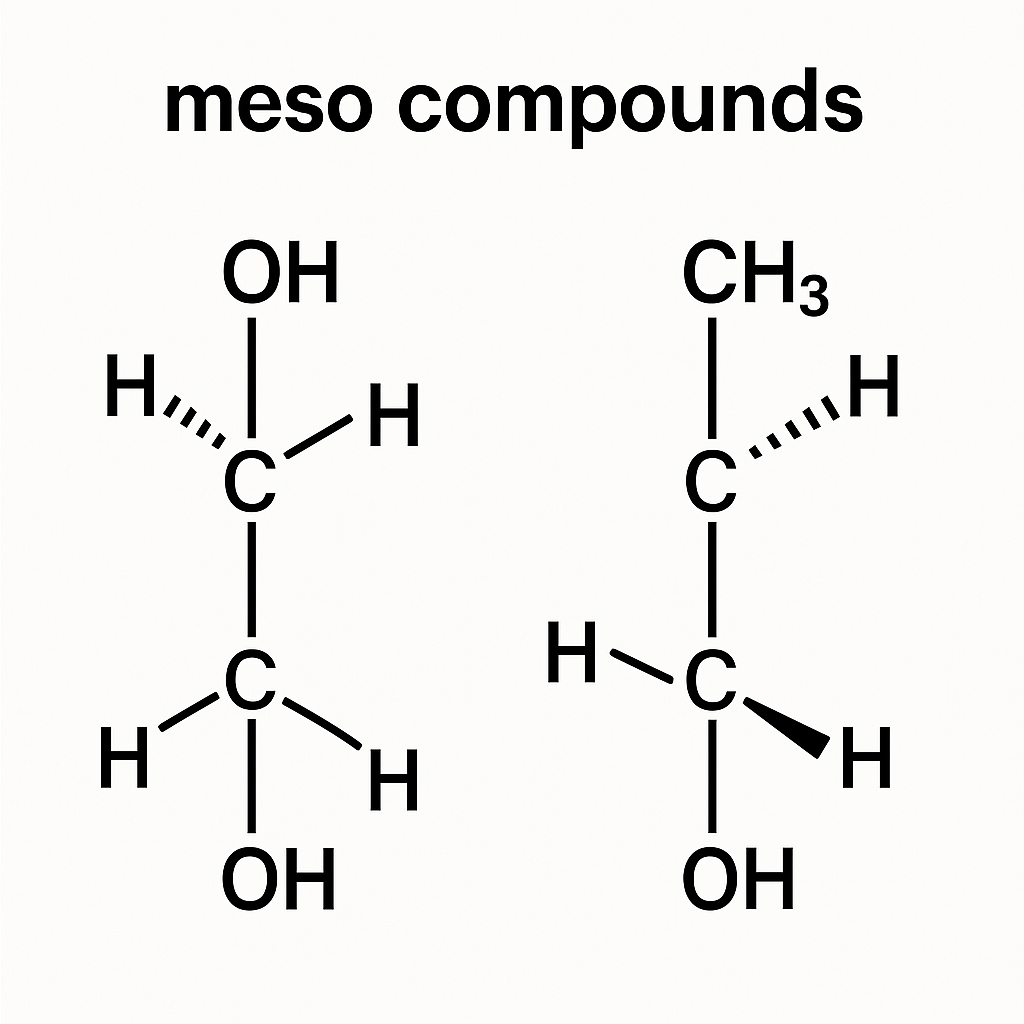
Understanding Chirality vs. Meso Structures
Chiral molecules are not superimposable on their mirror image and lack symmetry. Meso compounds are exceptions: despite having chiral centers, the molecule’s mirror halves are identical, making the compound achiral.
- Chirality: Non-superimposable on its mirror image, can form enantiomers.
- Meso Structures: Superimposable on its mirror image, considered achiral despite having stereocenters.
This often confuses students because it seems like the molecule should rotate light—it doesn’t.
How to Identify a Meso Compound
Step-by-Step:
- Understand the Definition: Meso compounds are those that have an internal plane of symmetry, which makes them achiral. This plane of symmetry can be identified by looking for a mirror image within the molecule.
- Identify Chiral Centers: Look for chiral centers, which are carbon atoms bonded to four different groups. These centers can make the molecule appear chiral initially.
- Check for Symmetry: Determine if there is an internal plane of symmetry that includes at least one chiral center. This plane should split the molecule into two mirror-image halves.
- Verify Achirality: If the molecule has an internal plane of symmetry that includes one or more chiral centers, it is considered a meso compound and is achiral.
- Consider Structural Formula: Draw the structural formula of the compound and look for the presence of a plane of symmetry. This can be done using wedge and dash structures to visualize the three-dimensional arrangement.
- Use Chemical Tools: Utilize software or databases to assist in identifying the symmetry and structure of the compound. Tools like Chemdraw or MestRenova can help in visualizing and confirming the presence of a meso compound.
- Consult Literature: Compare the structure with known meso compounds and consult literature for similar cases to confirm the identification.
Meso Compound Example: Tartaric Acid
Tartaric acid (HOOC–CH(OH)–CH(OH)–COOH) is a classic example.
- Has two chiral centers
- In its meso form, one center is R, and the other is S
- A plane of symmetry runs vertically through the molecule
- The molecule is optically inactive
Despite having chiral centers, this form of tartaric acid is not optically active, confirming its meso status.
Other Examples of Meso Compounds
- Cis-1,2-dimethylcyclohexane: This compound has two chiral centers at the methyl-substituted carbons, but the presence of a plane of symmetry makes it a meso compound.
- 1,4-Dimethylcyclohexane: Another example where the internal symmetry prevents it from being optically active, despite having multiple chiral centers.
| Compound | Key Features |
|---|---|
| 2,3-Butanediol | Two chiral centers; meso form is symmetrical |
| 1,2-Dichloro-1,2-ethanediol | Symmetrical across central C–C bond |
| Meso-2,4-pentanediol | Opposite stereochemistry at both chiral centers |
| Cis-1,2-dihydroxycyclohexane | Symmetrical in ring structure |
Meso vs. Racemic Mixtures
Definition and Characteristics
- Racemic Mixture: A racemic mixture, or racemate, is an equimolar mixture of two enantiomers that are mirror images of each other. This type of mixture is optically inactive because the effects of the two enantiomers cancel each other out, resulting in no net rotation of plane-polarized light.
- Meso Compound: A meso compound is a specific type of isomer that is also optically inactive but has a different structural reason for its lack of optical activity. Meso compounds have a symmetry element, such as a plane of symmetry, that makes them optically inactive despite being composed of chiral centers.
Formation and Separation
- Racemic Mixtures: These can form when a racemic resolution process is not fully effective, or when a chiral synthesis does not produce a single enantiomer. Techniques like chiral chromatography or crystallization can be used to separate enantiomers from a racemic mixture.
- Meso Compounds: These can arise from certain chemical reactions that produce symmetrical products. Separating a meso compound from its enantiomers can be more challenging because of its inherent symmetry, but techniques like chiral shift reagents in NMR spectroscopy can help differentiate between enantiomers in the presence of a meso compound.
Applications and Implications
- Racemic Mixtures: Often, racemic mixtures are used in applications where the specific activity of one enantiomer is not crucial, such as in some pharmaceuticals where the effects of the enantiomers are similar. However, in cases where enantiomeric purity is important, separating the enantiomers is necessary.
- Meso Compounds: Meso compounds are less common in practical applications but are important in understanding stereochemistry. They help in the study of molecular symmetry and the behavior of chiral molecules.
| Feature | Meso Compound | Racemic Mixture |
|---|---|---|
| Chirality | Achiral | Mixture of two enantiomers |
| Optical Activity | Optically inactive | Optically inactive (due to cancellation) |
| Physical Mixture? | Single compound | 50:50 mixture of enantiomers |
| Symmetry | Has internal symmetry | No symmetry; cancellation due to mixing |
Racemic mixtures are made of two chiral molecules with opposite rotation, while meso compounds are a single achiral molecule with built-in symmetry.
FAQs
Yes. As long as the molecule has an internal mirror plane and the stereocenters offset each other, it can still be meso.
No. Only those with internal symmetry and matching mirror-image halves are meso.
They are optically inactive, despite having stereocenters, because the symmetry cancels out their rotation of polarized light.
Yes. They are a type of diastereomer that is not optically active due to symmetry.
Use symmetry: if the compound has a plane of symmetry and is superimposable on its mirror image, it is meso—not chiral.
Conclusion
Meso compounds are fascinating exceptions in stereochemistry. Although they contain multiple chiral centers, their internal symmetry renders them achiral and optically inactive. Identifying meso compounds requires careful attention to both molecular structure and stereochemistry, but once you understand the pattern, they become much easier to recognize.
Understanding meso compounds helps reinforce broader concepts of chirality, symmetry, and stereoisomerism, making them essential for students studying organic chemistry or preparing for exams like the MCAT or GRE Chemistry.
To get detailed scientific explanations of Meso Compounds , try Patsnap Eureka.


