
What Are Valence Electrons?
Valence electrons are the electrons in the outermost shell of an atom, which participate in chemical bonding. These electrons determine the chemical properties of an element, including its reactivity and ability to form compounds with other elements. The number of valence electrons an atom has can be determined by looking at its position in the periodic table; for example, main-group elements typically have one to eight valence electrons, while transition metals can have up to ten.
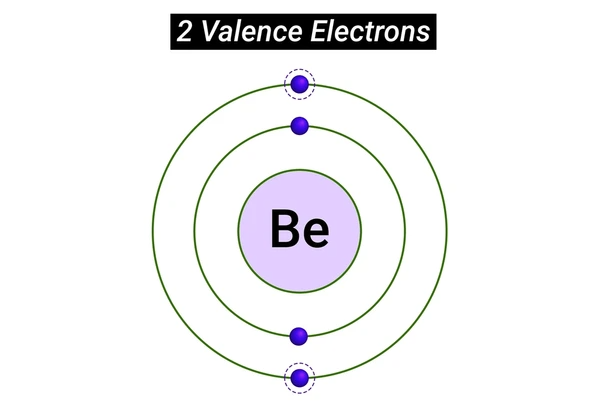
How to Identify Valence Electrons
- For Main Group Elements: Valence electrons are those in the outermost principal energy level. For example, carbon (C) has four valence electrons (one in each of its four 2p orbitals), while sodium (Na) has one valence electron (in its single 3s orbital).
- For Transition Metals: The identification can be more complex due to the presence of partially filled d and f subshells. For instance, iron (Fe) has six valence electrons (two in the 4s orbital and four in the 3d orbitals).
Role of Valence Electrons in Chemical Bonding
- Formation of Bonds: Valence electrons are involved in the formation of chemical bonds. In covalent bonds, atoms share valence electrons to achieve a stable electron configuration. For example, in a hydrogen molecule (H₂), two hydrogen atoms share their valence electrons.
- Electrostatic Attractions: In ionic bonds, valence electrons are transferred from one atom to another, resulting in the formation of ions that are electrostatically attracted to each other. For example, in sodium chloride (NaCl), a sodium atom loses its valence electron to become a Na⁺ ion, and a chlorine atom gains an electron to become a Cl⁻ ion.
- Bond Order and Stability: The number of valence electrons can influence the bond order and stability of a molecule. A higher number of valence electrons can lead to more complex bonding patterns and potentially more stable molecules.
Properties Determined by Valence Electrons
Chemical Properties
- Reactivity: Valence electrons are involved in chemical bonding and reactions. The number and arrangement of valence electrons determine how a material reacts with other elements. For instance, the reactivity of metals, nonmetals, and metalloids is primarily due to the number of valence electrons they have.
- Bonding: The type of chemical bonds formed (ionic, covalent, metallic) is directly influenced by the valence electrons. For example, metals tend to lose valence electrons to form positive ions, while nonmetals tend to gain electrons to form negative ions.
Electronic Properties
- Conductivity: The ability of a material to conduct electricity is largely determined by the valence electrons. In metals, free valence electrons are responsible for high electrical conductivity. In contrast, insulators have tightly bound valence electrons that do not contribute to conductivity.
- Electron Mobility: The ease with which valence electrons can move within a material affects its electrical and thermal conductivity. Higher mobility of valence electrons generally results in better conductivity.
Optical Properties
- Absorption and Reflection: The interaction of light with valence electrons determines a material’s optical properties, such as its color, transparency, and reflectivity. For example, the absorption of certain wavelengths of light can lead to coloration in materials.
- Luminescence: Certain materials emit light when valence electrons transition from higher to lower energy states, which is the basis for phenomena like phosphorescence and fluorescence.
Topological Properties
- Electronic Band Structure: The arrangement of valence electrons in energy bands affects the material’s electronic band structure, which is crucial for understanding its electrical properties and behavior under different conditions.
- Topological Insulators: Materials with specific arrangements of valence electrons can exhibit topological properties, such as the presence of topologically protected surface states, which are of interest for applications in quantum computing and spintronics.
Periodic Trends and Valence Electrons
- Electronegativity: Increases across a period and decreases down a group. This affects the polarity of bonds formed by an atom.
- Atomic Radius: Decreases across a period and increases down a group. This affects the strength of ionic and covalent bonds.
- Ionization Energy: Decreases down a group and increases across a period. This affects an atom’s ability to lose or gain electrons.
- Electron Affinity: Generally increases across a period and decreases down a group. This affects an atom’s ability to gain electrons.
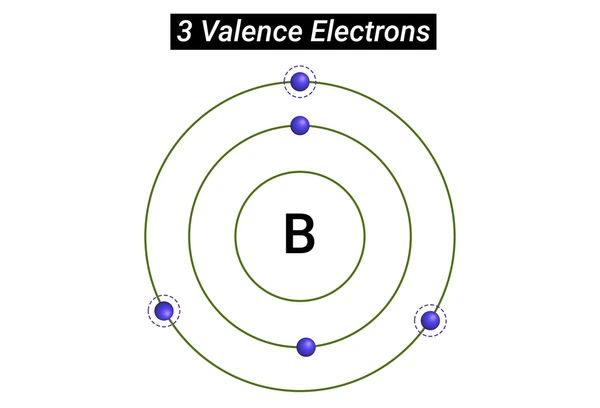
Examples of Valence Electrons Behavior
Bond Formation and Chemical Reactions
- Valence electrons participate in forming chemical bonds between atoms. For instance, in the formation of water (H₂O), two hydrogen atoms share their valence electrons with one oxygen atom, resulting in a covalent bond.
- In ionic bonding, valence electrons are transferred from one atom to another, leading to the formation of ions that are electrostatically attracted to each other, as seen in the formation of sodium chloride (NaCl).
Electronic Structure and Material Properties
- The arrangement of valence electrons influences the physical properties of materials. For example, in metals like aluminum, the free movement of valence electrons (surrendered by the metal atoms) is responsible for its high electrical and thermal conductivity.
- In semiconductors, the behavior of valence electrons determines their ability to conduct electricity, which is crucial for their application in electronics and photovoltaic cells.
Surface Properties and Friction
- The valence electron concentration affects the surface properties of materials, such as friction. In quasicrystals and B2-type approximants, the friction coefficient decreases with increasing valence electron-to-atom ratio, reaching a minimum at a specific value.
Dynamics and Excitations
- The dynamics of valence electrons can lead to excitations and transitions between different energy states. For instance, in potassium, large deviations from jellium behavior are observed due to the presence of d-type states above the Fermi level, leading to specific interband excitations.
Valence Electrons Importance in Everyday Chemistry
- Understanding valence electron behavior is essential for designing new materials with specific properties, such as increased strength, ductility, or stability, by manipulating the valence electron structures through alloying.
- The optical and electronic properties of materials, including their chemical reactivity, are largely determined by the behavior of valence electrons. Techniques like laser picoscopy can directly image these electrons at a picometer scale, enabling the study and manipulation of these properties.
- In advanced fields like nanotechnology, the behavior of valence electrons is being explored to develop new technologies, such as atomic magnets, where the magnetic properties are significantly influenced by the valence electrons of lanthanide elements.
Applications of Valence Electrons
Chemical Bonding and Materials Science
Valence electrons are crucial in forming chemical bonds between atoms, which is essential for creating materials with specific properties. For instance, metals have free valence electrons that contribute to their electrical conductivity and malleability.
Electronics and Semiconductors
In semiconductor technology, the arrangement of valence electrons determines the electrical conductivity of materials. Understanding valence electrons is vital for designing transistors and other semiconductor devices that form the basis of modern electronics.
Nuclear Magnetic Resonance (NMR) Spectroscopy
In NMR spectroscopy, valence electrons influence the magnetic properties of atoms, which is used to determine the structure of molecules. This technique is widely used in organic chemistry and pharmaceutical research.
Catalysis
Catalysts often work by providing or accepting valence electrons to facilitate chemical reactions. For example, in catalytic converters used in vehicles, valence electrons are involved in the reduction of pollutants such as nitrogen oxides and carbon monoxide.
Surface Science and Catalysis
The arrangement of valence electrons at the surface of materials can significantly affect their reactivity and catalytic properties. This is crucial in designing catalysts for industrial processes.
Optoelectronics
In materials like semiconductors and conductive polymers, the behavior of valence electrons is responsible for their optical properties, such as light emission and absorption. This is the basis for technologies like LEDs and solar cells.
Nanotechnology
At the nanoscale, the behavior of valence electrons can be manipulated to create materials with unique properties. This is used in developing nanomaterials for applications in electronics, medicine, and energy storage.

Latest Technical Innovations in Valence Electrons
Nanotechnology
Valence electrons are instrumental in the development of nanotechnology. At the nanoscale, the behavior of valence electrons can be manipulated to create materials with unique properties. For instance, the manipulation of valence electrons in carbon nanotubes has led to the creation of materials with exceptional strength, conductivity, and thermal stability.
Quantum Computing
In the field of quantum computing, valence electrons are used to create qubits, the fundamental units of quantum information. By leveraging the spin states of valence electrons, researchers have developed quantum bits that can exist in multiple states simultaneously, enabling quantum computing to perform complex calculations beyond the capabilities of classical computers.
Advanced Catalysis
Valence electrons are crucial in the development of advanced catalytic materials. By controlling the arrangement and behavior of valence electrons, researchers have created catalysts that can enhance chemical reactions under milder conditions, reducing energy consumption and environmental impact. For example, transition metal catalysts with precisely controlled valence electron configurations have shown improved efficiency in catalyzing hydrogenation reactions.
Optoelectronics
In optoelectronics, valence electrons are used to create materials with specific optical properties. The manipulation of valence electrons in semiconductors has led to the development of light-emitting diodes (LEDs), lasers, and photovoltaic cells. These devices rely on the ability to control the flow of valence electrons to emit or absorb light efficiently.
Energy Storage
Valence electrons are essential for the development of advanced energy storage systems. In batteries and supercapacitors, the movement of valence electrons between different materials enables the storage and release of electrical energy. Recent innovations have focused on materials with high valence electron mobility, leading to the development of batteries with higher energy densities and faster charging times.
Surface Science
In surface science, the behavior of valence electrons at the surface of materials is studied to understand and control surface properties. This knowledge is used to develop materials with specific surface characteristics, such as corrosion resistance, biocompatibility, and catalytic activity. Techniques like photoelectron spectroscopy are used to study the valence electron states at surfaces.
To get detailed scientific explanations of valence electrons, try Patsnap Eureka.

