
Polyprotic acids are a vital category of acids in chemistry, characterized by their ability to donate more than one proton (H⁺) per molecule in aqueous solution. Unlike monoprotic acids like HCl, polyprotic acids undergo multiple ionization steps, each with its own equilibrium constant. These stepwise dissociations add complexity to acid-base chemistry, especially in pH calculations and buffer design.
What are polyprotic acids? Eureka Technical Q&A explains that polyprotic acids can donate more than one proton (H⁺) in solution—like sulfuric or phosphoric acid—requiring stepwise pH calculations and finding use in buffering systems, fertilizers, and industrial processes.
In this article, we explain the structure and types of polyprotic acids, show how to perform pH calculations, and explore practical applications in industrial, environmental, and biological contexts.
What Are Polyprotic Acids?
Polyprotic acids are acids that can donate two or more protons (hydrogen ions) per molecule. Each ionization occurs sequentially, with the acid dissociating one proton at a time in water. Every ionization step has a distinct acid dissociation constant (Ka).
General Reaction:
HₙA ⇌ H⁺ + Hₙ₋₁A⁻ ⇌ H⁺ + Hₙ₋₂A²⁻ … (and so on)
Each successive proton is harder to remove, meaning:
- Ka₁ > Ka₂ > Ka₃
Types of Polyprotic Acids
1. Diprotic Acids
Can donate two protons.
- Examples: Sulfuric acid (H₂SO₄), Carbonic acid (H₂CO₃), Oxalic acid (H₂C₂O₄)
2. Triprotic Acids
Can donate three protons.
- Examples: Phosphoric acid (H₃PO₄), Citric acid (C₆H₈O₇)
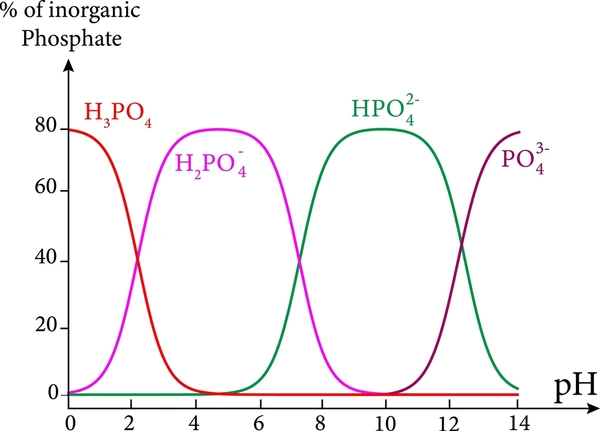
Ionization Steps and Ka Values
Example: Phosphoric Acid (H₃PO₄)
- H₃PO₄ ⇌ H⁺ + H₂PO₄⁻ Ka₁ ≈ 7.1 × 10⁻³
- H₂PO₄⁻ ⇌ H⁺ + HPO₄²⁻ Ka₂ ≈ 6.3 × 10⁻⁸
- HPO₄²⁻ ⇌ H⁺ + PO₄³⁻ Ka₃ ≈ 4.5 × 10⁻¹³
Observation: The first proton ionizes more readily than the second, and the third is even less likely to dissociate.
pH Calculations for Polyprotic Acids
- The number of ionizable protons
- The relative values of Ka₁, Ka₂, etc.
- The acid concentration
Case 1: Strong First Ionization
If Ka₁ is large (as in H₂SO₄), the first ionization is complete, and pH can be estimated using the initial concentration.
Example: 0.1 M H₂SO₄
- First step: fully dissociates → [H⁺] = 0.1 M
- Second step: calculate additional [H⁺] using Ka₂
Case 2: Weak Polyprotic Acid (e.g., H₂CO₃)
Only the first ionization significantly contributes to [H⁺]. Subsequent dissociations are often negligible in pH calculations for dilute solutions.
Stepwise approach:
- Use ICE table for Ka₁ to calculate [H⁺].
- Use resulting concentrations in second ICE table for Ka₂.
Buffering Capacity of Polyprotic Acids
- Each ionization step offers a distinct buffering region.
- Their conjugate base pairs can resist changes in pH at multiple pKa values.
Example: Phosphoric Acid
- Buffer at pH ≈ 2.1 (H₃PO₄/H₂PO₄⁻)
- Buffer at pH ≈ 7.2 (H₂PO₄⁻/HPO₄²⁻)
- Buffer at pH ≈ 12.3 (HPO₄²⁻/PO₄³⁻)
Real-World Applications
1. Biological Systems
- Carbonic acid (H₂CO₃) regulates blood pH via the bicarbonate buffer system.
- Phosphoric acid is crucial in ATP (adenosine triphosphate) and cellular energy transfer.
2. Buffer Solutions
- Used in biochemistry labs, pharmaceuticals, and diagnostic devices.
3. Industrial Processes
- Sulfuric acid is used in battery production, fertilizer manufacturing, and mineral processing.
- Citric acid is used as a preservative and pH buffer in food and beverage industries.
4. Environmental Chemistry
- Acid rain contains diprotic acids like sulfurous and sulfuric acid, which affect soil and aquatic ecosystems.
- Polyprotic acids are important in the treatment of wastewater and industrial effluents.
Summary Table: Common Polyprotic Acids
- Citric Acid: A widely known polyprotic acid used in various applications, including food and medicine.
- Carbonic Acid: Formed in solution by the reaction of carbon dioxide with water.
- Sulfuric Acid: A strong polyprotic acid that can donate up to two protons per molecule.
- Phosphoric Acid: Another strong polyprotic acid, which can donate up to three protons per molecule.
| Acid | Type | Ionization Steps | Approximate pKa Values |
|---|---|---|---|
| Sulfuric acid | Diprotic | 2 | -3 (Ka₁), 1.9 (Ka₂) |
| Carbonic acid | Diprotic | 2 | 6.3 (Ka₁), 10.3 (Ka₂) |
| Phosphoric acid | Triprotic | 3 | 2.1, 7.2, 12.3 |
| Citric acid | Triprotic | 3 | 3.1, 4.8, 6.4 |
| Oxalic acid | Diprotic | 2 | 1.2, 4.2 |
Conclusion
Polyprotic acids are essential to understanding acid-base equilibria, especially when multiple protons can dissociate from a single molecule. Their stepwise ionization, pKa values, and buffering abilities make them central to both theoretical and applied chemistry. Whether you’re calculating pH, preparing a buffer, or studying biological pathways, mastering polyprotic acid is a fundamental step in your chemistry journey.
FAQs
It can donate more than one proton (H⁺) in solution.
Monoprotic acids release only one proton per molecule, while polyprotic acids release two or more in multiple steps.
No. Typically, only the first dissociation significantly affects pH unless the solution is highly concentrated.
Each ionization step provides a conjugate acid-base pair that can resist changes in pH.
Not necessarily. Some like sulfuric acid have a strong first ionization, followed by weaker steps.
To get detailed scientific explanations of polyprotic acids, try Patsnap Eureka.


