
A single replacement reaction, also called a single displacement reaction, is a type of chemical reaction where one element replaces another in a compound. These reactions are common in both laboratory and real-world chemical processes, including metal extraction, corrosion, and electrochemical reactions.
This article explains the definition, general equation, how to predict outcomes, and examples of single replacement reactions in everyday and industrial chemistry.
Definition of a Single Replacement Reaction
What is a single replacement reaction? Eureka Technical Q&A explains that it’s a type of chemical reaction where one element replaces another in a compound, typically following the pattern A + BC → AC + B, commonly seen in metals and halogens.
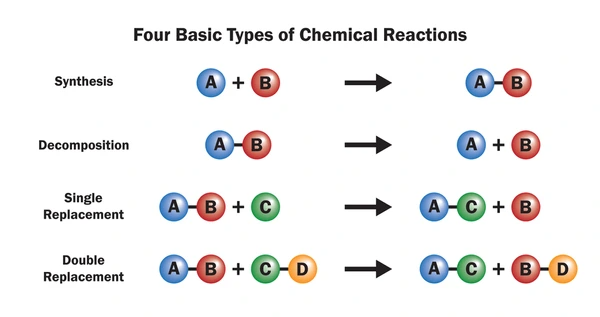
A single replacement reaction occurs when a more reactive element replaces a less reactive element in a compound. The general form is:
A + BC → AC + B
- A = a free element (usually a metal or halogen)
- BC = a compound containing B and C
- AC = a new compound
- B = displaced element (which becomes uncombined)
This type of reaction usually happens between metals or halogens and often takes place in aqueous solutions.
Types of Single Replacement Reactions
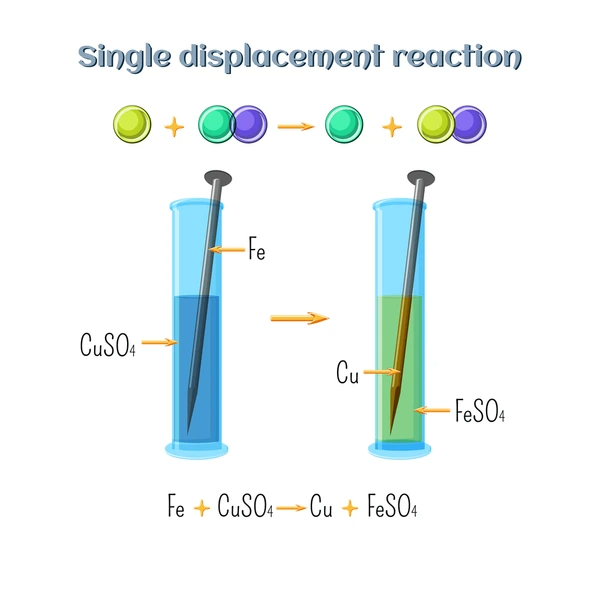
1. Metal Replacing Metal
A more reactive metal displaces a less reactive metal from its salt solution.
Example:
Zn + CuSO₄ → ZnSO₄ + Cu
(Zinc replaces copper because it’s more reactive.)
2. Metal Replacing Hydrogen
Active metals can replace hydrogen in acids or water.
Example (acid):
Mg + 2HCl → MgCl₂ + H₂
(Magnesium replaces hydrogen in hydrochloric acid.)
Example (water):
2Na + 2H₂O → 2NaOH + H₂
(Sodium reacts with water to release hydrogen gas.)
3. Halogen Replacing Halogen
A more reactive halogen displaces a less reactive halogen from its compound.
Example:
Cl₂ + 2KBr → 2KCl + Br₂
(Chlorine replaces bromine due to higher reactivity.)
The Activity Series
To predict whether a single replacement reaction will occur, chemists use an activity series, which ranks elements by their reactivity.
- A reaction only occurs if the free element is more reactive than the element it attempts to replace.
- If the free element is less reactive, no reaction happens.
Example:
Cu + ZnSO₄ → no reaction
(Copper is less reactive than zinc, so it cannot displace it.)
Real-World Applications
- Metal Extraction in Chemistry: Single displacement reactions play a crucial role in the extraction of metals. For instance, zinc can displace other metals in a compound. This principle is utilized in processes like the extraction of gold and silver from their ores, where zinc is used to replace these metals in their compounds.
- Construction and Material Science: In the construction industry, single replacement reactions can occur when saltwater comes into contact with concrete pillars made of iron. The iron reacts with chloride ions from the saltwater, forming iron (II) chloride and releasing hydrogen gas. This reaction can lead to the deterioration of the iron structures over time.
- Art Conservation: An interesting example of a single replacement reaction in everyday life is the oxidation of the Statue of Liberty. The statue’s exterior is made of copper, which reacts with the air and moisture to form a green patina layer. This reaction helps protect the underlying copper from further oxidation and corrosion.
- Everyday Life: Single replacement reactions are also found in various household items and processes. For example, the tarnish on silverware can be removed by reacting it with a zinc-based cleaner. The zinc displaces silver from the silver sulfide compound, restoring the shine to the silver.
Application Cases
| Product/Project | Technical Outcomes | Application Scenarios |
|---|---|---|
| PGM Recovery Process Johnson Matthey | Efficient recovery of platinum group metals using selective single replacement reactions | Recycling of catalytic converters and electronic waste |
| Cementation Process Teck Resources | High-purity copper recovery from mine drainage using iron-based single replacement reaction | Copper mining and environmental remediation |
| Electrocoat Primer PPG Industries | Enhanced corrosion resistance through controlled single replacement reactions on metal surfaces | Automotive and industrial coating applications |
| Gold Recovery System Newmont Corporation | Improved gold extraction efficiency using zinc-based single replacement reactions | Gold mining and precious metal recovery operations |
| E-Scrap Recycling Technology Mitsubishi Materials | Selective recovery of rare earth elements from electronic waste using tailored single replacement reactions | Electronics recycling and sustainable resource management |
FAQs
A single replacement reaction involves one element replacing another. In a double replacement, two compounds exchange ions to form new compounds.
Signs include color change, gas release, or solid formation, and the displaced element appearing separately.
The reactivity of elements varies. A less reactive element cannot displace a more reactive one from a compound.
Yes. Halogens often undergo such reactions based on their relative reactivity.
Not always. Some occur at room temperature, while others need heating or a catalyst.
Conclusion
A single replacement reaction is a fundamental chemical process where one element displaces another in a compound based on their reactivity. These reactions are key to understanding redox chemistry, industrial synthesis, and everyday chemical behavior. By using the activity series and recognizing reactivity trends, you can predict and understand when and why these reactions occur.
How PatSnap Eureka Accelerates Innovation in Single Replacement Reactions
Single replacement reactions are fundamental to redox chemistry, metallurgy, and materials synthesis. PatSnap Eureka equips researchers with powerful tools to explore how this reaction type is being applied, optimized, and patented across various industries.
- Patent Intelligence: Eureka scans global patent databases to surface innovations involving single replacement reactions, including novel redox systems, metal recovery processes, and synthetic applications.
- Competitive Landscape Tracking: Discover how organizations are utilizing single replacement reactions in fields such as battery technology, corrosion control, and inorganic compound synthesis.
- Trend Forecasting: Eureka’s AI-driven insights reveal emerging trends in single replacement applications, from green chemistry initiatives to next-generation manufacturing techniques.
- Technical Clustering: Visual clustering highlights key areas of research, such as reactive metal systems, ion exchange processes, and environmentally friendly reagent development.
With PatSnap Eureka, teams can accelerate discovery and innovation by unlocking deep, actionable insights into the evolving role of single replacement reactions in modern chemistry.
To get detailed scientific explanations of Single Replacement Reaction, try Patsnap Eureka.


