
What Is Aluminum Bromide?
Aluminum bromide (AlBr₃) is a highly reactive chemical compound that plays a significant role in both organic synthesis and industrial processes. Known for its ability to act as a Lewis acid catalyst, aluminum bromide is essential in reactions like Friedel-Crafts alkylation and acylation. Its reactivity and hygroscopic nature make it both useful and hazardous, requiring careful handling in laboratories and industrial settings.
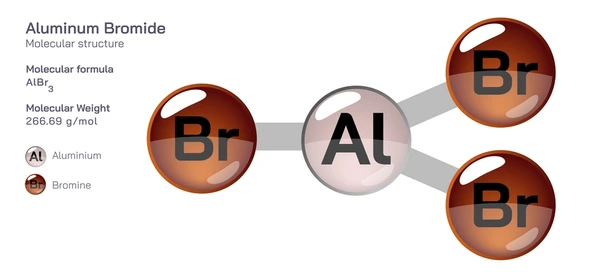
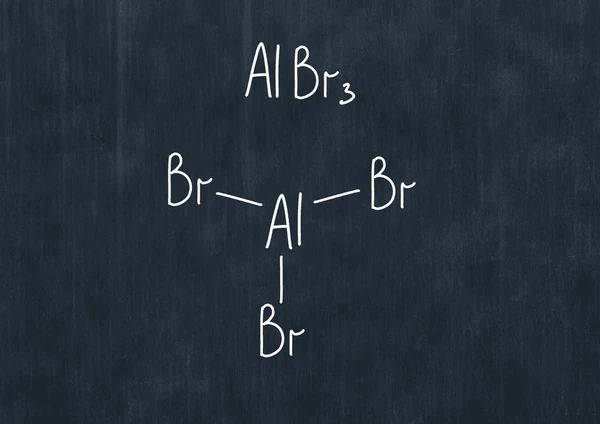
Structure and Properties of Aluminum Bromide
Crystal Structure of Aluminum Bromide (AlBr3)
Aluminum bromide (AlBr3) crystallizes in a hexagonal close-packed structure, with aluminum atoms occupying two-thirds of the octahedral holes formed by bromine atoms. The crystal structure can be described as a distorted tetrahedral arrangement of bromine atoms around the central aluminum atom.
Chemical Properties
- Lewis Acidity: AlBr3 is a strong Lewis acid, capable of accepting electron pairs from Lewis bases. This property makes it useful as a catalyst in various organic reactions, such as Friedel-Crafts alkylation and acylation.
- Reactivity: AlBr3 readily reacts with water, alcohols, and amines, forming aluminum hydroxide, alkoxides, and amido complexes, respectively. It also reacts with hydrogen bromide to form aluminum bromide complexes.
- Thermal Stability: AlBr3 is thermally stable and can be heated to temperatures up to 400°C without decomposition, making it suitable for high-temperature applications.
- Coordination Behavior: AlBr3 can form a variety of coordination complexes with various ligands, exhibiting different coordination geometries, such as tetrahedral, square pyramidal, and trigonal bipyramidal.
Synthesis of Aluminum Bromide
Electrochemical Preparation
Aluminum bromide can be prepared electrochemically in a cell with an aluminum anode and a hydrogen bromide solution in an aprotic solvent as the electrolyte. The reaction proceeds without additional electrical power after initiation. The product is a solution or solid aluminum bromide depending on the solubility limit.
Direct Synthesis from Elements
Aluminum bromide can be synthesized directly by reacting aluminum metal with bromine gas at elevated temperatures, typically 100-400°C. The aluminum bromide produced can have high purity of >99%.
Halide Exchange Reactions
Aluminum bromide can be obtained by halide exchange reactions between aluminum chloride and hydrogen bromide or other bromide sources like boron tribromide. This involves passing aluminum chloride vapors over molten aluminum at high temperatures.
Reaction Mechanisms
- Electrochemical route: At the anode, aluminum is oxidized to Al3+ which combines with bromide ions from the hydrogen bromide electrolyte to form AlBr3.
- Direct synthesis: Aluminum metal reacts with bromine in an oxidation-reduction reaction to form aluminum bromide.
- Halide exchange: AlCl3(g) + 3HBr(g) → AlBr3(g) + 3HCl(g)
Safety and Handling Precautions
Toxicity and Health Hazards
- Toxicity: Aluminum bromide is highly toxic if ingested, inhaled, or if it comes into contact with the skin. It can cause severe irritation to the respiratory tract, skin, and eyes.
- Health Hazards: Prolonged exposure can lead to serious health issues, including respiratory problems, skin burns, and eye damage. It can also cause systemic toxicity if absorbed into the bloodstream.
Fire and Explosion Hazards
- Flammability: Aluminum bromide is not highly flammable, but it can react violently with water, releasing toxic fumes such as hydrogen bromide and aluminum oxide.
- Explosion Hazards: It can be a fire hazard if involved in a combustion reaction, and it may explode if subjected to high temperatures or physical shock.
Environmental Hazards
- Environmental Impact: Aluminum bromide is harmful to aquatic life if released into water bodies. It can cause long-term adverse effects in the aquatic environment.
Handling and Storage Precautions
- Personal Protective Equipment (PPE): When handling aluminum bromide, it is essential to wear appropriate PPE, including gloves, safety goggles, and a lab coat. In cases of large-scale handling, a respirator may also be necessary.
- Storage Conditions: Store aluminum bromide in a cool, dry place, away from incompatible substances such as water and strong oxidizers. It should be kept in tightly sealed containers made of materials that are resistant to bromine attack.
Spill and Disposal Procedures
- Spill Response: In case of a spill, evacuate the area and ventilate the space. Use inert absorbent materials to contain and clean up the spill, and dispose of the waste according to local regulations.
- Disposal: Dispose of aluminum bromide in accordance with local environmental protection regulations. It should not be disposed of in regular waste streams or in the environment.
First Aid Measures
- Inhalation: Move the affected individual to fresh air immediately. If breathing is difficult, administer oxygen and seek medical attention.
- Skin Contact: Wash the affected area with plenty of water for at least 15 minutes. Remove contaminated clothing and seek medical attention if irritation persists.
- Eye Contact: Rinse the eyes with plenty of water for at least 15 minutes, lifting upper and lower eyelids occasionally. Seek medical attention immediately.
- Ingestion: Do not induce vomiting. Rinse the mouth with water and seek immediate medical attention.
Applications of Aluminum Bromide
Friedel-Crafts Reactions
AlBr3 is widely used as a catalyst in Friedel-Crafts alkylation and acylation reactions, facilitating the introduction of alkyl or acyl groups onto aromatic rings. Its selectivity is advantageous in certain cases.
Halogenation Reactions
AlBr3 can act as a brominating agent, promoting the formation of carbon-bromine bonds in organic compounds. It can convert alkyl halides into more brominated derivatives, such as ethylenic dibromide, acetylene tetrabromide, and hexabromethane.
Ether Cleavage
AlBr₃ effectively cleaves ether bonds, transforming ethers into new functional groups or removing protective groups.
Rearrangement Reactions
AlBr₃ catalyzes rearrangement reactions like Beckmann and Fries, facilitating the isomerization of organic compounds.
Electrochemical Synthesis
manufacturers produce AlBr₃ electrochemically from aluminum anodes and hydrogen bromide, enabling its use as an electrolyte or precursor.
Antiperspirant and Astringent Applications
Basic aluminum bromides, particularly the 5/6 basic form, exhibit excellent astringent properties and compatibility with cosmetic solvents, making them suitable for use in antiperspirant and deodorant formulations.
Catalytic Polymerization
AlBr3 solutions can act as catalysts for polymerization reactions, such as olefin polymerization, enabling the synthesis of polymeric materials.
Application Cases
| Product/Project | Technical Outcomes | Application Scenarios |
|---|---|---|
| Aluminium Bromide Catalysed Friedel-Crafts Reactions | Enables selective alkylation and acylation of aromatic compounds with high regioselectivity and improved yields compared to traditional methods. | Synthesis of fine chemicals, pharmaceuticals, and agrochemicals requiring precise control over product distribution. |
| Aluminium Bromide Bromination | Facilitates the formation of carbon-bromine bonds, enabling the synthesis of brominated organic compounds with enhanced reactivity and versatility compared to their non-brominated counterparts. | Production of brominated intermediates for further synthetic transformations, such as in the manufacture of pharmaceuticals, agrochemicals, and specialty chemicals. |
| Aluminium Bromide Ether Cleavage | Selectively cleaves ether linkages, allowing for the removal of ether protecting groups or the transformation of ethers into other functional groups with high chemoselectivity. | Synthetic organic chemistry, particularly in the synthesis of complex natural products and pharmaceuticals, where ether cleavage is a key step. |
| Aluminium Bromide Rearrangement Catalysis | Enables various rearrangement reactions, such as the Beckmann and Fries rearrangements, facilitating the isomerisation of organic compounds with high selectivity and efficiency. | Synthesis of fine chemicals, pharmaceuticals, and agrochemicals where rearrangement reactions are crucial for obtaining desired product structures. |
| Electrochemical Synthesis of Aluminium Bromide | Enables the sustainable and cost-effective production of aluminium bromide through electrochemical methods, reducing the environmental impact and waste generation associated with traditional synthesis routes. | On-site production of aluminium bromide for use in various chemical processes, minimising transportation and storage requirements. |
Latest Technical Innovations of Aluminum Bromide
Electrochemical Synthesis and Aluminum Production
- Electrochemical preparation of AlBr3 from aluminum anode and HBr electrolyte solution, enabling continuous production without additional power
- Novel multistep process using AlBr3 as an intermediate to produce metallic aluminum from aluminum-containing ores
- Electrolysis of AlBr3 melt to deposit aluminum at the cathode, with bromine recycling
Catalytic and Synthetic Applications
- Use of AlBr3 as a powerful brominating agent for alkyl halide synthesis and rearrangements
- Friedel-Crafts reactions and ether cleavage catalyzed by AlBr3
- Synthesis of aluminum hydrides (e.g. AlH3) and aluminum borides (e.g. AlB12) from AlBr3
Stabilized AlBr3 Solutions and Complexes
- Stabilized AlBr3 solutions in cyclohexane using benzene or triphenol additives for improved shelf life
- Alcohol-soluble AlBr3 complexes with zinc or zirconium for aerosol and antiperspirant formulations
- Recyclable AlBr3 complexes with alkyl esters for catalytic applications like olefin polymerization
Emerging Applications
- Aluminum electroplating from AlBr3-based molten salt baths for corrosion protection and complex geometries
- Stabilized alkyl bromide compositions containing AlBr3 for degreasing and cleaning applications
- Graphite intercalation compounds with AlBr3 and bromine for potential energy storage and catalysis
Technical Challenges of Aluminum Bromide
| Electrochemical Synthesis of AlBr3 | Developing efficient and continuous electrochemical processes for the production of AlBr3 from aluminum anodes and HBr electrolyte solutions, without requiring additional power input. |
| Aluminum Production from AlBr3 | Designing novel multistep processes that utilise AlBr3 as an intermediate to produce metallic aluminum from aluminum-containing ores, with efficient bromine recycling. |
| Catalytic Applications of AlBr3 | Exploring the use of AlBr3 as a powerful brominating agent and catalyst for various organic syntheses, such as alkyl halide synthesis, rearrangements, Friedel-Crafts reactions, and ether cleavage. |
| Stabilised AlBr3 Solutions | Developing stabilised AlBr3 solutions with improved shelf life and solubility, using additives like benzene, triphenol, or complexing agents like zinc or zirconium compounds. |
| Synthesis of Aluminum Hydrides and Borides | Utilising AlBr3 as a precursor for the synthesis of aluminum hydrides (e.g., AlH3) and aluminum borides (e.g., AlB12), with potential applications in hydrogen storage and advanced materials. |

FAQs:
- What is aluminum bromide used for?
Aluminum bromide is primarily used as a Lewis acid catalyst in organic synthesis, such as in Friedel-Crafts reactions. It’s also used in bromination processes. - Is aluminum bromide hazardous?
Yes, aluminum bromide is highly reactive with moisture and can release hydrogen bromide gas, which is toxic and corrosive. Proper safety measures are essential. - How is aluminum bromide made in the lab?
It’s prepared by reacting aluminum metal with bromine gas in a dry, moisture-free environment. - What happens when aluminum bromide reacts with water?
It hydrolyzes rapidly, producing hydrobromic acid (HBr) and aluminum hydroxide, which makes it highly reactive in humid conditions. - Can aluminum bromide be used in organic chemistry?
Absolutely! It’s widely used as a catalyst in organic reactions, particularly for facilitating bond formation in complex synthesis.
To get detailed scientific explanations of the aluminium bromide, try Patsnap Eureka.

Learn more
Hypertonic vs. Hypotonic vs. Isotonic: What’s the Difference?
Acetophenone: A Key Compound in Fragrance and Industry
Magnesium Nitrate: Key Uses, Definition, and Innovations
