
Enolates are reactive intermediates in organic chemistry that play a central role in forming carbon–carbon bonds. They are formed by deprotonating the α-hydrogen of carbonyl compounds and serve as key nucleophiles in a variety of synthetic reactions. Enolate chemistry underpins classic transformations such as aldol condensations, alkylation, and Michael additions, making it indispensable in complex molecule construction.
This article provides a comprehensive guide to enolate formation, their reactivity, and the most important reactions involving enolates, including mechanisms, conditions, and real-world applications.
What Are Enolates?
Enolates are resonance-stabilized anions formed by removing a proton (typically acidic) from the α-carbon of a carbonyl compound. The negative charge is delocalized between the α-carbon and the oxygen atom.
General Formula:
R–C(=O)–CH₂⁻ ⇌ R–C(–O⁻)=CH₂
This resonance structure allows enolates to act as nucleophiles at both the α-carbon and oxygen sites.

Enolate Formation: Bases and Conditions
1. Strong Bases
Common bases used to generate enolates include:
- LDA (Lithium Diisopropylamide) – selective and non-nucleophilic
- NaH (Sodium Hydride) – strong base, releases H₂ gas
- NaOEt / NaOH – used under equilibrium conditions
2. Conditions
- Kinetic Enolate: Formed rapidly at low temperature with bulky bases (LDA, -78°C). Less substituted, more reactive.
- Thermodynamic Enolate: Formed under warm conditions with weaker bases (NaOEt), more substituted, more stable.
Aldol Reaction: Enolate in Action
The aldol reaction is a fundamental carbon–carbon bond-forming reaction where an enolate reacts with another carbonyl compound to form a β-hydroxy carbonyl (aldol product).
Mechanism:
- Enolate formation
- Nucleophilic attack on another carbonyl
- Protonation to give β-hydroxy compound
Overall Reaction:
R–CH₂–C(=O)–R’ + R’’–C(=O)–R’’’ → R–CH(OH)–CH₂–C(=O)–R’’’
Aldol Condensation:
When heated or under acidic/basic conditions, the aldol product dehydrates to form an α,β-unsaturated carbonyl compound.
Alkylation of Enolates
Enolates can undergo SN2 reactions with alkyl halides to form new C–C bonds at the α-position.
General Reaction:
R–CH₂–C(=O)–R’ → R–CH(R”)–C(=O)–R’
Conditions:
- Base: LDA or NaH
- Electrophile: Primary alkyl halides or tosylates
- Solvent: THF, DMSO, or aprotic polar solvents
Challenges:
- Over-alkylation: excess base or alkyl halide may add twice
- Side reactions: elimination or O-alkylation under poor conditions
Claisen Condensation
An enolate of an ester reacts with another ester to form a β-keto ester in this C–C bond-forming reaction.
Mechanism:
- Enolate formation from ester
- Nucleophilic attack on carbonyl carbon of another ester
- Elimination of alkoxide group
Example: Ethyl acetate + base → Ethyl acetoacetate (β-keto ester)
Michael Addition: Conjugate Addition of Enolates
Enolates can add to α,β-unsaturated carbonyl compounds in a 1,4-conjugate fashion — forming a stable C–C bond at the β-position.
Reaction:
R–CH₂–C(=O)–R’ + CH₂=CH–C(=O)–R” → Michael adduct
Key features:
- Mild conditions
- Selective for conjugate (1,4) addition over 1,2
Enamine Reactions: Enolate Alternatives
Enamines are nitrogen-based tautomers of enolates that react similarly in alkylation and acylation reactions. They are more stable and can be easily hydrolyzed after C–C bond formation.
Used in:
- Stork enamine synthesis
- Regioselective α-substitution of ketones
Applications of Enolate Chemistry
- Synthesis of Complex Molecules
Enolates play a central role in forming complex organic compounds. They enable key reactions like aldol, Michael addition, and Mannich reactions, which help build carbon-carbon and carbon-heteroatom bonds. - Asymmetric Synthesis
Chemists use enolate chemistry to create chiral centers in asymmetric synthesis. Metal enolates—such as lithium, boron, or copper—support enantioselective reactions to yield optically active compounds, essential in pharmaceuticals. - Silicon Compound Formation
Silicon enolates allow scientists to synthesize complex silicon frameworks and photoinitiators. These structures are useful in advanced materials and electronics. - Catalysis Applications
Enolates act as catalysts or precursors in various reactions. For example, copper-catalyzed enolate reactions can produce cyclopentanone derivatives used in organic synthesis. - Pharmaceutical Intermediate Synthesis
Enolates help produce intermediates for beta-lactams and tetracycline analogs. These compounds serve as building blocks for antibacterial and antifungal drugs. - Fluorination Reactions
Fluorinating enolates is vital for developing fluorinated drugs and agrochemicals. Transition metal complexes often assist in these enantioselective fluorination reactions. - Material Science and Engineering
Enolates contribute to creating specialized materials, including polymers and nanomaterials. Their ability to form stable metal complexes makes them valuable for deposition techniques in material science.
Common Enolate Reactions at a Glance
- Aldol Reactions: This involves the addition of the enol or enolate form of a carbonyl compound to an aldehyde or ketone. Aldol reactions are crucial for carbon-carbon bond formation and can be highly diastereoselective, especially with chiral auxiliaries or catalysts .
- Enolate Alkylation: Enolates react with alkyl halides to form carbon-carbon bonds. This reaction is often used to synthesize complex molecules and can be diastereoselective, especially when chiral reagents or catalysts are employed .
- Claisen Condensation: This is a specific type of aldol reaction where two esters react to form a β-keto ester. It is a key reaction in the synthesis of ketones from esters .
- Enolate Coupling Reactions: Enolates can participate in coupling reactions with various electrophiles, including aldehydes, ketones, and Michael acceptors. These reactions are valuable for constructing carbon-carbon bonds in natural product synthesis .
- Enolate Trapping Reactions: Enolates can be trapped by electrophiles such as carbonyl compounds, leading to the formation of new carbon-carbon bonds. This is often used in synthetic strategies to build complex molecular frameworks .
| Reaction Type | Key Product | Notes |
|---|---|---|
| Aldol Reaction | β-hydroxy carbonyl | Reversible, leads to C–C bond |
| Aldol Condensation | α,β-unsaturated carbonyl | With heat or acid/base |
| Alkylation | α-alkylated carbonyl | SN2 route with alkyl halides |
| Claisen Condensation | β-keto ester | Ester version of aldol |
| Michael Addition | 1,5-dicarbonyl compound | Selective 1,4-addition |
| Enamine Alkylation | α-alkylated carbonyl | Mild and regioselective |
Conclusion
Enolates are versatile, highly reactive intermediates that enable essential carbon–carbon bond-forming reactions in organic synthesis. Whether used in aldol reactions, alkylation, or Michael additions, their chemistry is foundational to both laboratory and industrial-scale transformations. Mastering enolate formation and reactivity is essential for chemists designing complex molecules, pharmaceuticals, and functional materials.
FAQs
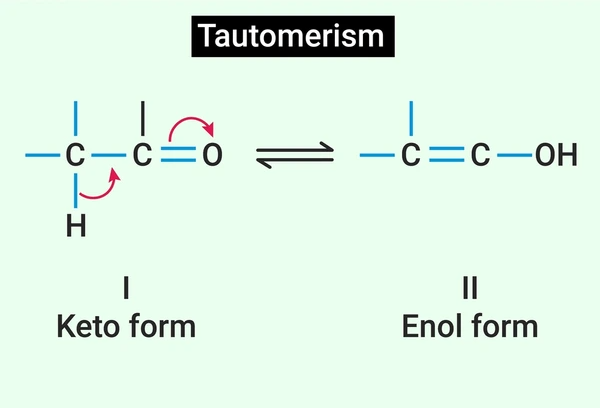
An enol is a neutral tautomer of a carbonyl compound with a C=C–OH structure, while an enolate is the deprotonated, negatively charged intermediate formed by removing an α-hydrogen.
Strong, non-nucleophilic bases like LDA or NaH are ideal for complete and selective enolate formation.
Kinetic enolate: Less substituted, forms faster at low temperature.
Thermodynamic enolate: More stable, forms at higher temperature with weaker bases.
Yes, especially under basic conditions, esters form enolates which participate in Claisen condensations.
It enables selective C–C bond formation, crucial in the synthesis of complex organic molecules, drugs, and materials.
To get detailed scientific explanations of enolates, try Patsnap Eureka.


