Acid-base chemistry is one of the most fundamental and versatile concepts in science. Over the decades, different models have been developed to describe how acids and bases behave—starting from Arrhenius to Brønsted-Lowry and later Lewis theory. Among these, the Brønsted-Lowry acid-base theory, proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923, provided a crucial expansion by redefining acids not just in water, but across solvents and systems. This model forms the backbone of our understanding in biochemistry, organic synthesis, catalysis, and even environmental science.
This article will help explore the latest chemical structures, patents, and technological applications of Brønsted acids in seconds through PatSnap Eureka AI Agent.
What Is a Brønsted-Lowry Acid?
In simple terms, a Brønsted-Lowry acid is a proton donor—a molecule or ion that can release a hydrogen ion (H⁺) in a chemical reaction. Unlike the earlier Arrhenius theory (which restricted acids to those producing H⁺ in water), Brønsted-Lowry acids are defined by their proton-transferring behavior, making the theory more versatile across a wide range of chemical environments.
Key Characteristics
- Proton Donation: The ability to donate a proton is central to being a Brønsted-Lowry acid. This can occur in various contexts, such as in a reaction with a base or in the presence of water.
- Conjugate Acid-Base Pairs: When a Brønsted-Lowry acid donates a proton, it forms a conjugate base. The acid and its conjugate base differ by the presence or absence of a proton. For example, if acid HA donates a proton to become A⁻, then HA and A⁻ constitute a conjugate acid-base pair.
- Strength of Acids: The strength of a Brønsted-Lowry acid refers to its tendency to donate a proton. This is often quantified by the acid dissociation constant (Ka), which represents the equilibrium constant for the dissociation of the acid in water. A higher Ka value indicates a stronger acid. For instance, HCl has a Ka of 1 × 10³, making it a strong acid, while CH₃CO₂H has a Ka of 1.8 × 10⁻⁵, making it a weak acid.

Mechanism of Brønsted-Lowry Acids
Fundamental Principle: Proton Transfer
A Brønsted-Lowry acid acts by donating a proton (H⁺) to a Brønsted-Lowry base, which accepts it. This process doesn’t involve complex intermediates or bond rearrangements—just the movement of a proton from one molecule to another.
The hydrogen ion (H⁺) is essentially a bare proton—it has no electrons and is extremely reactive. In solution, it is almost never free-floating; instead, it attaches itself to a base, forming a new species (e.g., H₃O⁺ in water).
General Reaction Mechanism
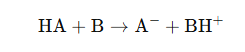
Where:
- HA is the Brønsted acid (proton donor)
- B is the Brønsted base (proton acceptor)
- A⁻ is the conjugate base (after losing H⁺)
- BH⁺ is the conjugate acid (after gaining H⁺)
This reaction is usually:
- Fast and reversible
- Influenced by electronegativity, solvent, and acid/base strength
Proton Transfer Steps (Example in Water)
Reaction:
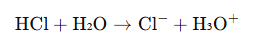
Step-by-step Mechanism
- HCl donates a proton (H⁺) to water.
- Lone pair on H₂O attracts the proton, forming H₃O⁺.
- Cl⁻ is left behind as the conjugate base.
Visual:
- Electron pair from water’s oxygen → attacks the hydrogen of HCl
- H–Cl bond breaks heterolytically (electrons stay with Cl)
- Proton attaches to water → H₃O⁺ forms
This is a one-step mechanism, often described as a concerted proton transfer.
Transition State & Energy Profile
Although it’s a simple proton shift, the reaction still has a transition state where:
- The proton is partially bonded to both the acid and base
- Some bond-breaking and bond-making occurs simultaneously
Reaction Coordinate Diagram:
- Shows a small energy barrier for weak acids
- Strong acids have low activation energy for dissociation
Applications of Brønsted Acids
Biological Systems
- Buffer systems in blood rely on carbonic acid (H₂CO₃) and bicarbonate (HCO₃⁻)
- Enzyme catalysis often involves proton donation/acceptance
- ATP hydrolysis involves Brønsted acid-base interactions
Industrial Use
- Used in acid-catalyzed reactions (e.g., esterification, hydrolysis)
- Essential in battery technology (sulfuric acid in lead-acid batteries)
- Involved in pH-sensitive processes like dye synthesis or polymerization
Pharmaceuticals
- Understanding acidity helps design drug molecules with correct solubility and bioavailability
- Brønsted acid-base interactions are key to drug binding with enzymes or receptors
Comparison with Other Acid Theories
| Theory | Definition of Acid | Solvent Required? |
|---|---|---|
| Arrhenius | Produces H⁺ in water | Only aqueous |
| Brønsted-Lowry | Donates a proton (H⁺) | No |
| Lewis | Accepts an electron pair | No |
Common Brønsted Acids
- Hydrochloric acid (HCl)
- Sulfuric acid (H₂SO₄)
- Ammonium ion (NH₄⁺)
- Acetic acid (CH₃COOH)
PatSnap Eureka AI Agent Capabilities
The theoretical knowledge of Brønsted-Lowry acids is just the beginning. In modern R&D workflows, chemical innovation relies heavily on data intelligence.
Using PatSnap Eureka AI Agent, scientists can:
Analyze Patent Trends
- Search for Brønsted acids used in new pharmaceutical formulations, green catalysts, or energy storage
- Track competitors and identify leading institutions in acid-based innovation
Visualize Molecular Structures
- Compare proton-donating groups across analogs
- Discover structure-activity relationships (SAR)
Benchmark Compounds
- Evaluate how one acid performs compared to others in similar reactions or formulations
- Predict future developments in acid catalysts based on AI-driven insights
Example: Eureka can identify a surge in patent activity related to organocatalysts using Brønsted acids, especially in sustainable polymerization technologies.
Common Misconceptions
- Myth: All acids taste sour or are dangerous.
→ Not true—many acids are safe or weak (like citric acid in lemons). - Myth: Water is neutral only.
→ Water is amphoteric, meaning it can act as both a Brønsted acid and base. - Myth: Brønsted theory replaces Lewis theory.
→ These theories are complementary, each useful in different contexts.
Conclusion
The Brønsted-Lowry acid-base theory remains a cornerstone of modern chemistry. Its simplicity—proton donors and acceptors—makes it both elegant and widely applicable, from high school chemistry to high-tech labs.
When combined with tools like PatSnap Eureka AI Agent, this foundational concept becomes a gateway to real-world discovery—fueling innovation across pharmaceuticals, materials, green chemistry, and more.
Whether you’re analyzing a buffer system or scouting patents for the next generation of catalysts, understanding Brønsted-Lowry acids puts you ahead in both theory and practice.
FAQs
Q: Can a substance be both a Brønsted acid and base?
Yes. Water (H₂O), for example, can donate or accept a proton depending on the reaction.
Q: What’s the difference between strong acid and concentrated acid?
Strength refers to how completely an acid dissociates; concentration refers to how much acid is present per volume.
Q: Are all Brønsted acids corrosive?
No. Some weak acids (like carbonic or lactic acid) are even found in the human body.
To get detailed scientific explanations of the Brønsted acids, try Patsnap Eureka.