At the heart of every biochemical transformation lies a fleeting yet essential interaction: the enzyme-substrate complex. This transient pairing enables enzymes to recognize, bind, and convert substrates into biologically meaningful products—powering everything from digestion to DNA replication.
In pharmaceutical and biotech innovation, understanding this interaction unlocks opportunities to engineer better drugs, diagnostic tools, and industrial catalysts. With PatSnap Eureka AI Agent, life science professionals can explore enzyme-substrate interaction patterns, global patent trends, and IP strategies—accelerating the path from molecular insight to marketable innovation.
What is an Enzyme-Substrate Complex?
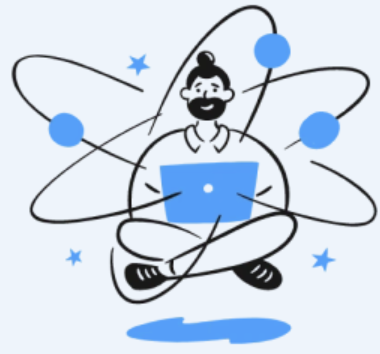
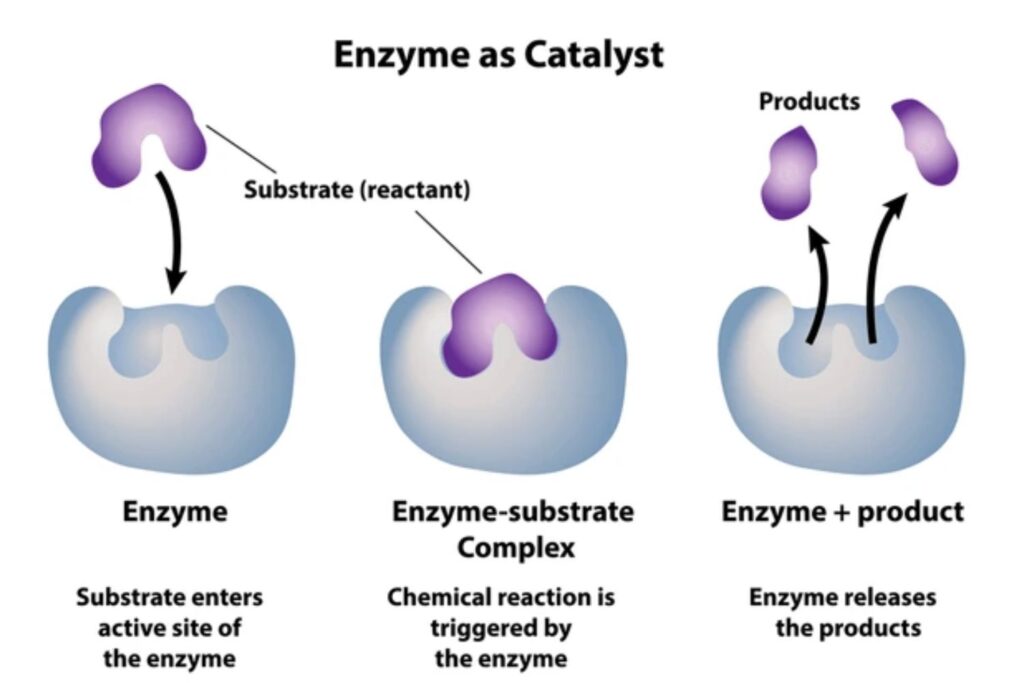
An enzyme-substrate complex (often abbreviated as ES complex) refers to the transient molecular assembly that forms when a substrate molecule binds to the active site of a specific enzyme. This complex is not a stable product, but rather an intermediate step in a larger biochemical reaction.
The formation of the ES complex is the crucial first step in enzymatic catalysis, which allows the enzyme to lower the activation energy required for the reaction to proceed. This makes the reaction faster and more efficient, often by factors of millions compared to an uncatalyzed version.
The ES complex plays a central role in enzyme kinetics, especially in the Michaelis-Menten model, which mathematically describes how reaction rate depends on substrate concentration. In this model:
E+S⇌ES→E+P
Where:
- E = enzyme
- S = substrate
- ES = enzyme-substrate complex
- P = product
The binding process is highly selective and governed by the three-dimensional complementarity between the enzyme’s active site and the substrate’s structure and charge distribution.
Key Characteristics of the Enzyme-Substrate Complex
1. Specificity
- Each enzyme is typically tailored to recognize and bind only one specific substrate or a small group of structurally similar substrates. This is driven by precise interactions such as hydrogen bonds, electrostatic forces, van der Waals interactions, and hydrophobic effects. This property ensures fidelity in metabolic pathways and drug action.
2. Reversibility
- The formation of the ES complex is reversible under normal physiological conditions. The substrate may bind and unbind several times before undergoing transformation. This dynamic equilibrium allows for fine regulation of enzymatic activity and contributes to the concept of Km (Michaelis constant), representing the substrate concentration at which the reaction rate is half its maximum.
3. Catalytic Function
- Within the ES complex, the enzyme often orients the substrate, positions functional groups, and stabilizes the high-energy transition state, significantly accelerating the reaction. This catalytic environment can involve acid-base catalysis, covalent catalysis, or metal ion coordination.
4. Transient Nature
- ES complexes are short-lived and exist only as intermediate states. The entire catalytic cycle, from substrate binding to product release, often occurs in milliseconds to seconds. This fleeting existence is crucial for enzyme turnover, allowing rapid processing of many substrate molecules.
Key Forms and Structural Models
Lock and Key Model
Proposed by Emil Fischer, this model suggests a perfect fit between enzyme and substrate—like a key fitting into a lock. It explains high specificity but does not account for enzyme flexibility.
Induced Fit Model
Proposed by Daniel Koshland, this model shows that enzymes undergo a conformational change to mold around the substrate. This dynamic interaction better reflects real biological conditions.
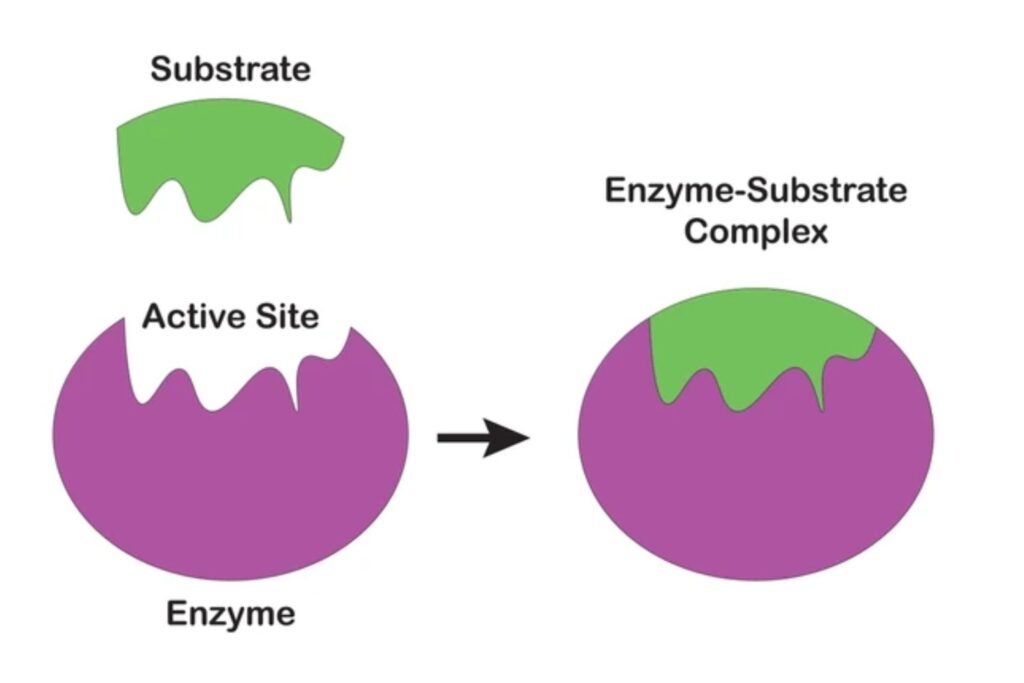
Mechanism of Action
- Recognition and Binding: The substrate binds to the enzyme’s active site, forming the ES complex.
- Catalysis: Enzyme stabilizes the transition state, reduces activation energy, and facilitates the reaction.
- Product Formation: Substrate is converted into product(s).
- Release: The product dissociates, and the enzyme is regenerated for another cycle.
This mechanism underscores the catalytic efficiency and specificity that make enzymes vital to biological and industrial processes.
Factors Affecting the Formation of the Enzyme-Substrate Complex
1. Substrate Concentration
- Higher substrate concentration increases the likelihood of collision and binding.
- Saturation point leads to maximum enzyme velocity (Vmax).
2. Enzyme Concentration
- More enzyme molecules increase available active sites, enhancing complex formation—up to a limit based on substrate availability.
3. pH Level
- Each enzyme has an optimal pH. Deviations can disrupt active site geometry and substrate binding.
4. Temperature
- Moderate increases in temperature raise kinetic energy and binding rate.
- Extreme heat denatures enzymes, reducing complex formation.
5. Inhibitors
- Competitive inhibitors block the active site.
- Noncompetitive inhibitors alter the enzyme’s conformation.
- Both reduce the formation of functional ES complexes.
6. Cofactors and Coenzymes
- Some enzymes require metal ions (e.g., Mg²⁺) or vitamins (e.g., NAD⁺) for optimal activity and substrate binding.
7. Allosteric Regulation
- Binding of a molecule at an allosteric site can increase or decrease substrate affinity at the active site.
Clinical and Industrial Applications
- Metabolic Pathways: Enzymes in metabolic pathways, such as glycolysis and the citric acid cycle, form ES complexes with their substrates to catalyze the reactions that break down nutrients and generate energy.
- DNA Replication and Repair: Enzymes involved in DNA replication and repair, such as DNA polymerases and ligases, form ES complexes with DNA to facilitate the synthesis and repair of the genetic material.
- Drug Metabolism: Cytochrome P450 enzymes, which are involved in the metabolism of many drugs, form ES complexes with their substrates to catalyze the oxidation of foreign compounds, making them more water-soluble and easier to excrete.
- Enzyme Inhibitors: Enzyme inhibitors, such as protease inhibitors used to treat HIV, work by binding to the active site of an enzyme and preventing the formation of the ES complex.
- Artificial Enzymes: Researchers are developing artificial enzymes that can form ES complexes with specific substrates to catalyze reactions that are not naturally occurring.
In Medicine
- Drug Targets: Enzymes like HIV protease or acetylcholinesterase are targeted by inhibitors that mimic substrates.
- Enzyme Therapy: Used in lysosomal storage disorders (e.g., Fabrazyme for Fabry disease).
- Diagnostics: Enzymes such as glucose oxidase enable blood glucose monitoring.
In Industry
- Food Tech: Lactase, proteases, and amylases enhance food processing and digestibility.
- Biofuels: Cellulases and lipases convert biomass into bioethanol or biodiesel.
- Green Chemistry: Enzymes offer cleaner alternatives to traditional chemical catalysts.
Innovation Landscape
The enzyme-substrate complex is being re-engineered through:
- Machine learning-based enzyme prediction
- Directed evolution and synthetic biology
- Immobilization techniques for reuse in reactors
- Allosteric site design for better control
With PatSnap Eureka AI Agent, you can:
- Monitor top-filing biotech firms
- Track emerging startups in enzyme R&D
- Analyze competitive filings in protein engineering
- Evaluate clinical pipeline overlaps for therapeutic enzymes
Comparison: Enzyme-Substrate Complex vs Similar Biological Systems
| Feature | Enzyme-Substrate Complex | Antibody-Antigen Complex | Receptor-Ligand Complex |
|---|---|---|---|
| Function | Catalysis | Immune recognition | Signal transduction |
| Duration | Very short-lived | Minutes to hours | Varies (short to long) |
| Specificity | High | Very high | Moderate to high |
| Reusability | Enzyme reused | Antibody can be consumed | Receptor usually reused |
Conclusion
The enzyme-substrate complex isn’t just a biochemical curiosity—it’s a gateway to controlling life processes, engineering innovation, and treating disease. Its fleeting nature hides profound implications for catalysis, specificity, and design.
Harnessing tools like PatSnap Eureka AI Agentenables researchers, IP teams, and life sciences professionals to extract value from the molecular level—identifying opportunities in enzyme innovation, tracking global patent activity, and accelerating time-to-market for new therapeutics and technologies.
FAQs
A short-lived structure where an enzyme binds to its substrate to catalyze a biochemical reaction.
Key factors include pH, temperature, substrate and enzyme concentrations, inhibitors, and cofactors.
Yes. Drug developers often design molecules to mimic or block substrate binding, affecting enzyme activity.
ES complex: Enzyme-Substrate complex, the initial binding before the reaction.
EP complex: Enzyme-Product complex, formed after the reaction but before the product is released.
The enzyme’s active site (lock) fits the substrate (key) precisely, showing high specificity—only the correct substrate can bind and trigger the reaction.
To get detailed scientific explanations of the enzyme-substrate complex, try Patsnap Eureka.