
What Is Geometric Isomerism?
Geometric isomerism—also called cis-trans isomerism—is a type of stereoisomerism found in molecules with restricted rotation around a bond, typically double bonds or cyclic structures. These isomers have the same molecular formula but differ in the spatial arrangement of atoms or groups. Although the difference may seem minor, geometric isomers can have dramatically different chemical properties, reactivity, and biological functions. This article explains what geometric isomerism is, how cis and trans isomers differ, and why understanding this concept is essential in organic chemistry and real-world applications.
What is geometric isomerism? Eureka Technical Q&A explains this type of stereoisomerism where molecules with the same formula differ in spatial arrangement around a double bond or ring, commonly seen as cis and trans forms in organic chemistry.
Geometric isomerism occurs when molecules have the same atoms connected in the same order but differ in how substituent groups are oriented around a rigid structure, such as a carbon–carbon double bond or a ring.
The rigidity of these structures prevents free rotation, which locks groups into specific positions relative to one another. As a result, two (or more) geometric isomers can exist with distinct physical and chemical characteristics.
Cis-Trans Isomerism Explained
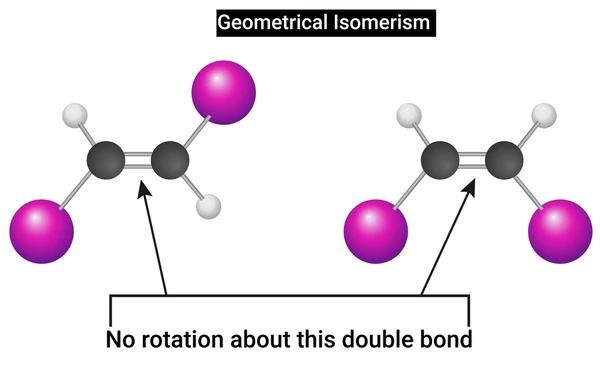
The most common type of geometric isomerism is cis-trans isomerism, found in alkenes and certain ring compounds.
Cis Isomer
In the cis isomer, two identical or similar groups are positioned on the same side of the double bond or ring. This often leads to higher polarity and lower boiling points due to uneven molecular shape.
Trans Isomer
In the trans isomer, the groups are located on opposite sides of the double bond or ring. This arrangement is generally less polar and may result in higher melting points and more linear geometry.
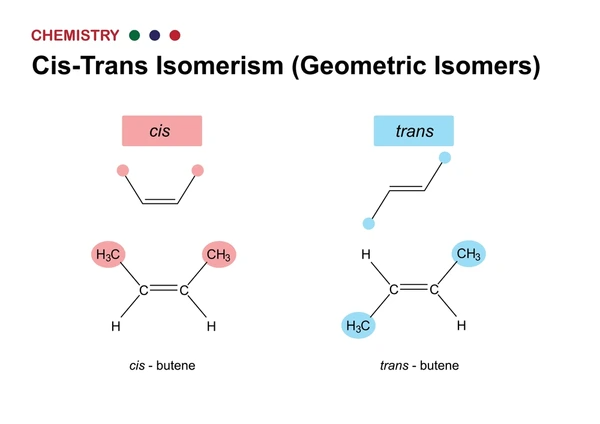
Example: 2-Butene
2-Butene (C₄H₈) is a classic example of geometric isomerism.
- Cis-2-butene: The two methyl (CH₃) groups are on the same side of the C=C bond.
- Trans-2-butene: The two methyl groups are on opposite sides of the C=C bond.
Both have the same molecular formula and connectivity, but their shapes and properties differ significantly.
Geometric Isomerism in Cyclic Compounds
Geometric isomerism also appears in cyclic structures, where ring strain prevents free rotation.
For example, in 1,2-dimethylcyclohexane, the two methyl groups can be either:
- Cis: Both on the same side of the ring (either both axial or both equatorial).
- Trans: One above and one below the plane of the ring.
This has implications in stereochemistry and conformational analysis, especially in organic synthesis and pharmaceutical design.
Conditions Required for Geometric Isomerism
For geometric isomerism to occur, three main conditions must be met:
- Restricted rotation around a bond (e.g., a double bond or ring structure).
- Different substituents on each end of the double bond or across the ring.
- No possibility of free rotation that would convert one isomer into the other without breaking bonds.
Physical and Chemical Property Differences
| Property | Cis Isomer | Trans Isomer |
|---|---|---|
| Polarity | Usually more polar | Less polar |
| Boiling Point | Higher (stronger dipole) | Lower (less dipole interaction) |
| Melting Point | Lower (less symmetrical) | Higher (more symmetrical) |
| Solubility | Often more soluble in polar solvents | Less soluble in polar solvents |
How to recognize the possibility of geometric isomerism
- Identify Double Bonds: Geometric isomerism, also known as cis-trans isomerism or E/Z isomerism, occurs primarily around double bonds. Look for carbon-carbon double bonds or other double bonds in the molecule.
- Check for Restricted Rotation: For geometric isomerism to be possible, the molecule must have some part that cannot rotate freely. This restriction can be due to the presence of rings, bulky groups, or other structural features that hinder rotation around a bond.
- Analyze the Substituents Around Double Bonds: Around each double bond, consider the arrangement of substituents (atoms or groups attached to the carbons of the double bond). The relative positions of these substituents can lead to different isomers.
- Look for Cis and Trans Isomers: If a molecule has a double bond with two different substituents on either side, it can exist as cis (both substituents on the same side) or trans (substituents on opposite sides) isomers. This is a common type of geometric isomerism.
- Consider Steric Hindrance: If the molecule has groups that are sterically hindered from rotating, this can also contribute to the possibility of geometric isomerism. Atropisomerism is a type of geometric isomerism resulting from such hindered rotation.
- Use Chemical Nomenclature: The terms “cis” and “trans” (or “E” and “Z”) are used in the IUPAC nomenclature to describe the relative positions of substituents around a double bond. If a molecule can be named using these terms, it indicates the possibility of geometric isomerism.
Real-World Applications of Geometric Isomerism
Pharmaceutical Applications
Drug developers study geometric isomers to ensure safety and efficacy. For example, thalidomide had two isomers with drastically different effects. One provided therapeutic benefits, while the other caused severe birth defects. This case emphasizes the need for strict isomer control in pharmaceuticals.
Materials Science and Engineering
Scientists use geometric isomerism to design new materials with tailored properties. By adjusting molecular arrangements, they create advanced polymers, catalysts, and coatings. These custom materials offer improved strength, conductivity, or reactivity for specific applications.
Chemical Synthesis and Reaction Design
Organic chemists rely on isomer awareness to guide efficient reactions. Geometric isomers can change the direction and outcome of chemical processes. With proper design, researchers improve yields, reduce waste, and create more selective synthesis routes.
Optoelectronic Device Performance
Engineers control molecular geometry to tune the optical behavior of materials. This precision helps optimize devices like OLEDs and solar cells. Geometric isomers affect light absorption and emission, improving brightness, efficiency, and energy output.
Catalysis and Industrial Processes
Catalyst designers manipulate geometry to boost selectivity and activity. Geometric isomers with specific shapes improve reaction control. This fine-tuning makes industrial processes faster, cleaner, and more efficient.
FAQs
What is the main cause of geometric isomerism?
Restricted rotation around a double bond or ring prevents atoms from switching positions freely, creating fixed spatial arrangements.
Is geometric isomerism the same as optical isomerism?
No. cis-trans isomerism relates to spatial positioning around a bond, while optical isomerism involves molecules that are mirror images and affect polarized light differently.
Can alkanes show geometric isomerism?cis-trans isomerism
Generally, no. Alkanes have single bonds, which allow free rotation, so geometric isomerism doesn’t apply unless in a ring structure.
How do you name cis-trans isomers?
Use the prefixes cis- and trans- before the molecule’s name. For complex molecules, the E/Z system (based on atomic priority) is used for precision.
Are all geometric isomers stable?
Most are, but some cis-isomers may be less stable due to steric hindrance between nearby groups.
Conclusion
Geometric isomerism is a fundamental concept in chemistry that explains how molecules with the same formula can behave differently based on their spatial arrangement. Whether it’s the subtle shift of a group across a double bond or the placement on a ring, these structural differences impact molecular polarity, physical properties, and biological activity.
Understanding cis-trans isomerism not only deepens your grasp of molecular behavior but also has practical relevance in drug design, food science, and material development.
To get detailed scientific explanations of geometric isomerism, try Patsnap Eureka.


