
What is the Hydronium Ion?
The hydronium ion (H3O+) is a fundamental player in aqueous chemistry, especially in acid-base reactions. Formed when a proton (H+) combines with a water molecule (H2O), the hydronium ion is central to understanding pH, acidity, and the behavior of acids in water. Also known as the oxonium or hydroxonium ion, it plays a key role in various chemical processes, making it an essential concept in both theoretical and applied chemistry. This article delves into the properties, formation, and significance of the hydronium ion in chemical reactions and beyond.
Chemical Structure and Properties of Hydronium Ion
The hydronium ion, commonly represented as H3O+, also exists in hydrated forms like H5O2+, H7O3+, and H9O4+. These variations are collectively denoted as (H2O)nH3O+, where n represents the number of water molecules surrounding the ion. Hydrated forms play a vital role in stabilizing the ion through strong hydrogen bonds, which are essential in aqueous environments. Notably, the hydronium ion forms complexes like the Zundel ion (H5O2+) and Eigen ion (H9O4+), which demonstrate its dynamic interactions with water. These forms contribute to the unique behavior of acids in water, underscoring the importance of the hydronium ion in chemical reactions.
How Does the Hydronium Ion Form?
Basic Chemistry of Formation
Water molecules naturally ionize, creating H3O+ and OH− ions through an equilibrium reaction: 2H2O↔H3O++OH−2H_2O \leftrightarrow H_3O^+ + OH^-. This process shows how water donates a proton to another molecule, generating positively charged H3O+ and negatively charged hydroxide ions. The role of hydrogen bonding is crucial in this process, as water molecules form up to four hydrogen bonds, providing the stability needed for proton transfer and ion formation.
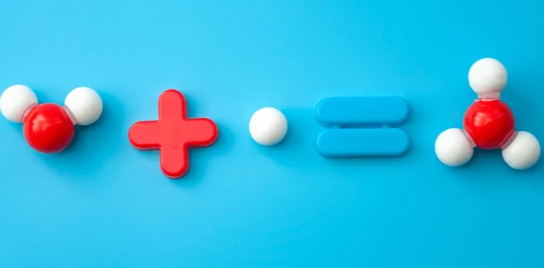
Structural Aspects
The molecular structure of H3O+ features a central oxygen atom bonded to three hydrogens in a trigonal pyramidal arrangement. In aqueous environments, this ion associates with additional water molecules, forming stable complexes like H5O2+, H7O3+, and H9O4+. These hydrated structures, held together by hydrogen bonds, are key to understanding proton behavior in water and its dynamic interactions with other molecules.
Formation Mechanisms
Proton transfer reactions, where a proton moves between water molecules, are central to the creation of H3O+. This movement relies on the intricate hydrogen bonding network in water, which supports efficient proton mobility. Formation can also occur on surfaces, such as silica, where water dissociates to create silanol groups and protons. This surface interaction underlines the diverse contexts in which proton-based ions are formed and stabilized.
Factors Influencing Ion Formation
- PH and Temperature: The concentration of H3O+ ions directly depends on the pH level of a solution. Lower pH values indicate higher concentrations, while higher pH levels suggest lower ion presence. Temperature also impacts water ionization, with elevated temperatures generally increasing the rate at which protons are transferred between molecules.
- Catalysts: The presence of certain catalysts significantly enhances proton formation. For instance, palladium or platinum catalysts can facilitate reactions like nitrate reduction with hydrogen, leading to the generation of hydroxylammonium ions, closely linked to proton activity in aqueous systems.
Applications of Hydronium Ion
- Chemical Reactions and Catalysis
Protons play a vital role in chemical reactions by interacting with hydrophilic molecular sites, especially areas rich in electron density. This capability is particularly significant in polymerization reactions. For example, in tetrahydrofuran (THF) polymerization, protons act as initiators, enhancing Poly-THF yields when combined with heteropolyacid catalysts. Additionally, they facilitate catalytic processes like hydrogenation and metal corrosion by balancing hydrogen molecules and ions in the presence of catalysts. - Biocidal Compositions
Proton sources are integral to biocidal formulations designed to combat biofilms. These compositions effectively deactivate and prevent biofilms in medical and industrial applications. By combining biocides with proton generators, these solutions offer targeted control over biofilm-related issues, improving hygiene and reducing risks. - Skin Conditioning and Wound Healing
Proton-enriched solutions contribute to skin hydration and wound healing. Formulations containing carbamide and protons promote amino acid synthesis, crucial for protein production and tissue repair. These solutions rehydrate the skin and support healing processes without the side effects of harsher medications. - Advanced Materials and Nanotechnology
Protons influence the sorption properties of materials like zeolites. Stable, hydrated protons within zeolite pores impact adsorption and interaction with organic molecules, altering pore volumes. This behavior is critical in catalysis and separation technologies, where molecular interactions define material performance. - Electrochemical Applications
Protons are essential in electrochemical systems, including hydrogen electrodes used for reference measurements and catalytic hydrogenation. These processes rely on maintaining equilibrium between hydrogen molecules and ions, enabling precise and efficient reactions in aqueous solutions. - Environmental and Hydrometallurgical Applications
Ion exchange processes involving protons have revolutionized metal recovery and purification. They enable efficient recovery of metals like gold and uranium and help purify cobalt solutions by removing impurities such as nickel and copper. This technology also supports water recycling, reducing reliance on freshwater resources in industrial processes. - Molecular Dynamics and Diffusion Studies
The diffusion behavior of protons in different media has been extensively studied through molecular dynamics simulations. For instance, their isotropic diffusion in hydrous oil dielectrics depends on water content, impacting applications like power transformer insulation. This knowledge aids in optimizing materials to minimize aging and enhance performance.
Application Cases
| Product/Project | Technical Outcomes | Application Scenarios |
|---|---|---|
| Skin Sanitizing and Wound Healing Solution Aphex Biocleanse Systems, Inc. | Highly protonated hydronium and carbamide technology, effective in sanitizing skin and promoting wound healing by addressing issues like harsh alcohol effects and limited effectiveness of other solutions. | Medical and personal care settings where skin sanitization and wound healing are critical. |
| Biocidal Compositions for Biofilm Control Ecolab USA, Inc. | Synergistic combination of biocides with hydronium ions, effective in deactivating, removing, and preventing biofilms, thus reducing microbial populations. | Industrial and medical environments where biofilm formation poses significant challenges. |
| Poly-Tetrahydrofuran Production HYOSUNG Corp. | Hydronium ions act as reaction initiators in the polymerization of tetrahydrofuran, improving the yield of Poly-THF when used with a heteropolyacid catalyst. | Chemical manufacturing processes requiring efficient polymerization reactions. |
| Hydrogen Halide Removal Process BASF SE | Utilizes hydronium ions in a rectifying column with a partial condenser to efficiently remove hydrogen halides, particularly hydrogen chloride. | Chemical industries where purification and separation of hydrocarbons are necessary. |
| Hydrous Oil Dielectric Study | Molecular dynamics simulations show that hydronium ions influence the diffusion behavior in hydrous oil dielectrics, indirectly indicating the synergism of water and acid in accelerating the aging of power transformer insulation materials. | Power transformer maintenance and aging studies, particularly in environments with high water content. |
Latest Technical Innovations in Hydronium Ion
- Catalysis and Reaction Mechanisms
Protons are pivotal in catalysis, significantly enhancing reaction rates and selectivity in various industrial and organic processes. Recent advancements in zeolite catalysts, which leverage protons, have improved hydrocarbon cracking efficiency. Innovations also emphasize eco-friendly catalytic processes, reducing reliance on harsh chemicals and promoting sustainable practices in green chemistry. - Electrochemical Applications
Protons are integral to advanced electrochemical systems, especially fuel cells and batteries. Recent progress in proton exchange membranes (PEMs) focuses on boosting conductivity and stability through advanced polymer blends and composites. These innovations improve fuel cell performance under diverse operating conditions. Novel proton-based batteries are being developed, offering promising energy densities and extended cycle lives, making them attractive for next-generation energy storage. - Materials Science and Hydrogels
Proton coordination plays a crucial role in creating innovative materials like hydrogels. Research into 3D-/4D-shape-morphing hydrogels has unlocked applications in soft robotics, drug delivery, and tissue engineering. These materials exhibit reversible shape changes, driven by precise ionic interactions, enhancing their versatility in advanced applications.
Technical Innovations and Performance Metrics
- Catalytic Systems:
Zeolite catalysts with enhanced proton exchange capabilities have shown higher reaction rates and improved selectivity in petrochemical processes, setting new benchmarks for catalytic efficiency. - Proton Exchange Membranes:
Cutting-edge PEMs achieve conductivity above 100 mS/cm at 80°C with remarkable chemical and mechanical stability, ensuring long-term functionality in fuel cells. - Proton-Based Batteries:
Emerging proton-ion batteries demonstrate energy densities up to 200 Wh/kg and exceed 1000 charge-discharge cycles, rivaling traditional lithium-ion alternatives.
Potential Applications
- Industrial Catalysis:
In petrochemicals, advanced zeolite catalysts have streamlined hydrocarbon cracking, improving process efficiency and reducing energy consumption. - Energy Sector:
Fuel cells powered by advanced PEMs are driving the shift toward cleaner energy, while proton-based batteries offer new possibilities for portable electronics and electric vehicles. - Biomedical Engineering:
Shape-morphing hydrogels driven by ionic interactions are revolutionizing biomedical applications, from precision drug delivery systems to innovative scaffolds for tissue engineering.
To get detailed scientific explanations of hydrogen ions, try Patsnap Eureka.

