Often referred to as the biochemical “powerhouse” of the cell, oxidative phosphorylation is the final and most efficient phase of cellular respiration. This process generates the majority of ATP—the universal energy currency essential for life. As such, oxidative phosphorylation plays a pivotal role in physiology, pathology, and drug development.
This article will help unravel the fundamental mechanics of energy production but also provides a window into mitochondrial diseases, metabolic syndromes, neurodegeneration, and even cancer therapies through PatSnap Eureka AI Agent. Researchers and pharmaceutical innovators can delve into the patent landscape, competitive research, and emerging technologies related to mitochondrial function and energy metabolism in Patsanp Eureka—accelerating discovery and strategic decisions.
What is Oxidative Phosphorylation?
Oxidative phosphorylation is the metabolic pathway through which cells use enzymes to oxidize nutrients, thereby releasing energy used to form adenosine triphosphate (ATP). This reaction takes place in the mitochondrial inner membrane and involves the electron transport chain (ETC) and ATP synthase.
It is the final stage of aerobic respiration, following glycolysis and the citric acid cycle, and is the main contributor to cellular ATP production.

Key Characteristics of Oxidative Phosphorylation
- Location: Occurs in the inner mitochondrial membrane
- Input Molecules: NADH and FADH₂ generated from earlier stages of respiration
- Output: ATP and water
- Mechanism: Involves a proton gradient created by electron transfer through protein complexes I–IV
- Energy Yield: Approximately 26–28 ATP molecules per glucose molecule
Key Molecular Complexes and Components
Electron Transport Chain (ETC)
The ETC comprises four protein complexes (I–IV) and two mobile carriers (ubiquinone and cytochrome c) that facilitate the transfer of electrons and proton pumping.
ATP Synthase (Complex V)
This rotary enzyme uses the proton motive force to synthesize ATP from ADP and inorganic phosphate (Pi).
Mechanism of Action: How It Works
Oxidative phosphorylation (OXPHOS) is a highly efficient process that generates ATP, the primary energy currency of the cell. It occurs in the inner mitochondrial membrane (IMM) of eukaryotic cells and is the final stage of aerobic respiration. The process can be divided into two main components: the electron transport chain (ETC) and chemiosmosis, which includes ATP synthase.
1. The Electron Transport Chain (ETC)
The ETC is a series of protein complexes (Complexes I-IV) and mobile electron carriers (ubiquinone and cytochrome c) embedded in the IMM. These complexes work together to transfer electrons from reducing agents (NADH and FADH2) to molecular oxygen (O2), the final electron acceptor.
- Complex I (NADH: Ubiquinone Oxidoreductase):
- Subunits: Approximately 45 subunits in mammals, including a flavin mononucleotide (FMN) cofactor and iron-sulfur (FeS) clusters.
- Function: Transfers electrons from NADH to ubiquinone (CoQ), reducing it to ubiquinol (CoQH2). It also pumps protons (H+) across the IMM.
- Inhibitors: Rotenone, piericidin A, and amobarbital.
- Complex II (Succinate: Ubiquinone Oxidoreductase):
- Subunits: 4 subunits in mammals, including a FAD cofactor and FeS clusters.
- Function: Transfers electrons from succinate to ubiquinone, reducing it to ubiquinol. It does not pump protons.
- Inhibitors: Carboxin, TTFA, and malonate.
- Complex III (Cytochrome bc1 Complex):
- Subunits: 11 subunits in mammals, including cytochromes b and c1, and an FeS cluster.
- Function: Transfers electrons from ubiquinol to cytochrome c. It also pumps protons across the IMM.
- Inhibitors: Antimycin A, myxothiazol, and stigmatellin.
- Complex IV (Cytochrome c Oxidase):
- Subunits: 13 subunits in mammals, including cytochromes a and a3, and copper centers (CuB and CuA).
- Function: Transfers electrons from cytochrome c to molecular oxygen (O2), reducing it to water (H2O). It also pumps protons across the IMM.
- Inhibitors: Cyanide, azide, and carbon monoxide.
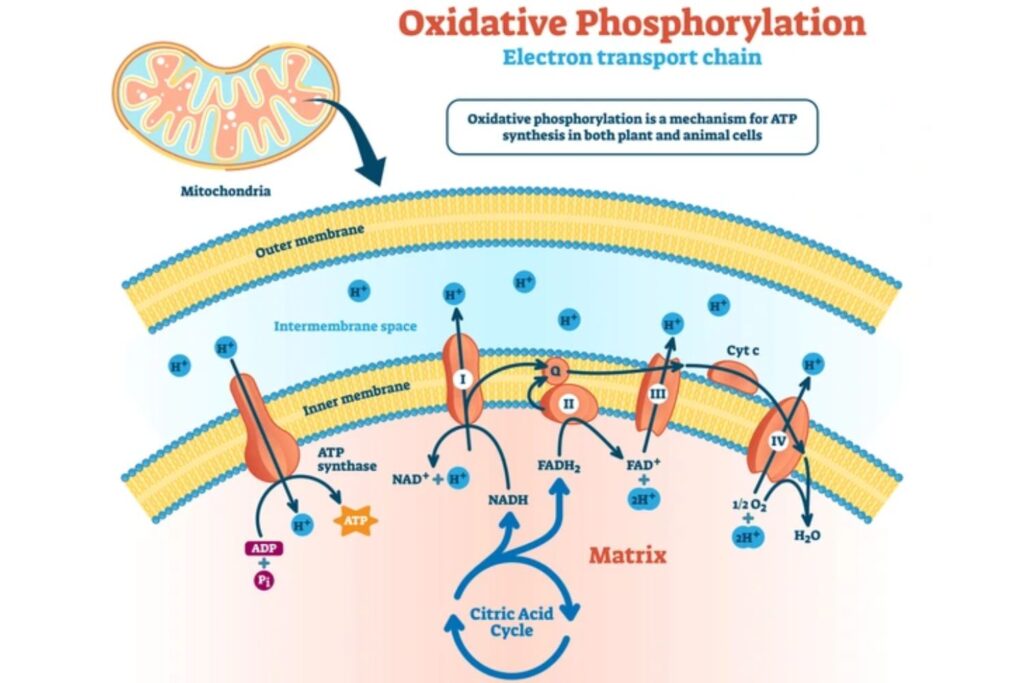
2. Chemiosmosis and ATP Synthesis:
The proton gradient established by the ETC drives ATP synthesis via chemiosmosis.
- ATP Synthase (Complex V):
- Structure: Composed of two main domains: F0 (membrane-bound, responsible for proton translocation) and F1 (peripheral, responsible for ATP synthesis).
- Mechanism: Protons flow back into the mitochondrial matrix through the F0 domain, causing conformational changes that drive the rotation of the F1 domain. This rotation catalyzes the synthesis of ATP from ADP and inorganic phosphate (Pi).
- Regulation: ATP synthase activity is regulated by the proton motive force (PMF), the concentration of ADP, and the presence of inhibitory proteins like IF1.
Clinical Relevance and Uses
Mitochondrial Diseases
Defects in oxidative phosphorylation are implicated in:
- Leigh Syndrome
- MELAS (Mitochondrial Encephalopathy, Lactic Acidosis, and Stroke-like episodes)
- Parkinson’s and Alzheimer’s diseases
Therapeutic Targeting
- Cancer Metabolism: Tumors with altered oxidative metabolism
- Anti-Aging Research: Caloric restriction and mitochondrial efficiency
- Cardiomyopathies: Mitochondrial dysfunction as a causative factor
Innovation: Patents, Trends & Technology Landscape
Oxidative phosphorylation is at the center of several innovative domains:
- Mitochondria-targeted antioxidants (e.g., MitoQ, SkQ1)
- OXPHOS inhibitors for oncology applications
- Gene therapy for mitochondrial repair
- CRISPR-mediated correction of mitochondrial DNA mutations
PatSnap Eureka AI Agent allows biotech companies, academics, and R&D teams to:
- Map global OXPHOS-related patent filings
- Benchmark technology leaders
- Uncover emerging startups focused on mitochondrial medicine
- Analyze licensing and acquisition opportunities
Side Effects and Safety Considerations
Disruption of oxidative phosphorylation can lead to:
- Increased reactive oxygen species (ROS)
- Cellular energy deficits
- Apoptosis and necrosis
Pharmacological targeting must balance efficacy with mitochondrial toxicity, especially in vulnerable tissues like the brain and heart.
Oxidative Phosphorylation vs Substrate-Level Phosphorylation
| Feature | Oxidative Phosphorylation | Substrate-Level Phosphorylation |
|---|---|---|
| Location | Mitochondrial inner membrane | Cytoplasm or mitochondrial matrix |
| Oxygen Requirement | Requires oxygen (aerobic) | Anaerobic or aerobic |
| Enzyme Involvement | Electron transport chain + ATP synthase | Kinase enzymes |
| ATP Yield per Glucose | High (26–28 ATP) | Low (2–4 ATP) |
| Efficiency | Highly efficient | Less efficient |
Conclusion
Oxidative phosphorylation is not only a fundamental bioenergetic process but also a strategic target in modern medicine and biotechnology. From treating rare mitochondrial diseases to developing novel anticancer therapies, understanding and modulating this process opens transformative possibilities.
With PatSnap Eureka AI Agent, life sciences professionals can decode the innovation landscape, pinpoint emerging IP, and align R&D with mitochondrial-focused breakthroughs—fueling smarter, faster innovation across academia and industry.
FAQs
It refers to the mitochondrial process that uses oxygen and the electron transport chain to generate ATP from ADP.
NADH typically yields about 2.5 ATP per molecule in eukaryotic cells under physiological conditions.
Oxidative phosphorylation is the process in mitochondria where ATP is synthesized using energy from electrons transferred to oxygen via the electron transport chain.
It produces over 90% of a cell’s ATP, supporting essential biological functions like muscle contraction, neurotransmission, and biosynthesis.
To get detailed scientific explanations of the Oxidative phosphorylation, try Patsnap Eureka.