
In organic and inorganic chemistry, sp³ hybridization is a fundamental concept that explains the tetrahedral geometry observed in molecules like methane (CH₄) and ethane (C₂H₆). This hybridization involves the mixing of one s-orbital and three p-orbitals to form four equivalent sp³ hybrid orbitals, each with 25% s-character and 75% p-character.
What is sp³ hybridization? Eureka Technical Q&A explains that sp³ hybridization occurs when one s orbital and three p orbitals mix to form four equivalent hybrid orbitals—creating a tetrahedral geometry, as seen in methane (CH₄) and many organic molecules.
What is SP³ Hybridization?
SP³ hybridization is a type of hybridization that occurs when one s orbital and three p orbitals of an atom mix to form four equivalent sp³ hybrid orbitals. This process is typically observed in molecules with tetrahedral geometry, such as methane (CH₄), where the carbon atom is bonded to four hydrogen atoms.
- One 2s orbital and three 2p orbitals (px, py, pz) combine.
- Four new sp³ hybrid orbitals are formed, each with identical energy and shape.
- These orbitals arrange themselves in a tetrahedral geometry to minimize repulsion (VSEPR theory).
Key Features of SP³ Hybrid Orbitals
- Shape: Asymmetric, lobed orbitals
- Bond angle: ~109.5° (ideal tetrahedral angle)
- Bond type: Single (σ) bonds
- Examples: Methane (CH₄), ethane (C₂H₆), ammonia (NH₃), water (H₂O)
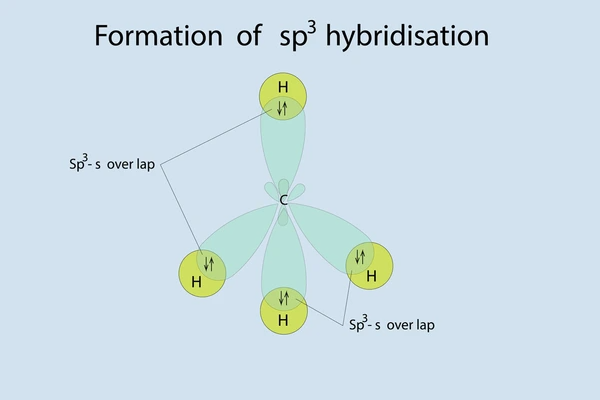
Tetrahedral Geometry and Bond Angles
The tetrahedral arrangement of sp³ hybrid orbitals ensures maximum separation between electron pairs, minimizing repulsion.
Ideal vs. Distorted Tetrahedral Geometry
Ideal Tetrahedral Geometry
- An ideal tetrahedron is a regular polyhedron with all equilateral edges and equilateral triangular faces. In mathematical terms, it is a convex polyhedron with four triangular faces, six straight edges, and four vertices.
- In hyperbolic geometry, an ideal tetrahedron is a tetrahedron whose vertices all lie at infinity. This means that the tetrahedron is not bounded and has infinite volume. Ideal tetrahedra are used in the study of hyperbolic 3-manifolds and are crucial in the gluing equations that describe these manifolds.
- The dihedral angles in an ideal tetrahedron are all equal to 70.53 degrees, and the shape parameters (complex numbers) associated with its edges determine its hyperbolic structure.
Distorted Tetrahedral Geometry
- A distorted tetrahedron deviates from the ideal shape due to various factors such as pressure, temperature changes, or impurities in the material. This distortion can lead to variations in edge lengths and angles, making the tetrahedron non-regular.
- The distortion can be quantified using indices such as the tetrahedral distortion index, which measures the deviation from the ideal tetrahedral geometry.
- Distorted tetrahedra are common in real materials and can affect the material’s properties, such as mechanical strength and thermal conductivity. For example, in quartz-like materials, the tetrahedral distortion is dependent on the chemical composition and can lead to significant changes in the material’s properties.
| Molecule | Hybridization | Bond Angle | Deviation Reason |
|---|---|---|---|
| CH₄ (Methane) | sp³ | 109.5° | Perfect tetrahedron |
| NH₃ (Ammonia) | sp³ | 107° | Lone pair repulsion |
| H₂O (Water) | sp³ | 104.5° | Two lone pairs |
- Lone pairs occupy more space than bonding pairs, compressing bond angles.
- Steric effects in larger molecules can further distort angles.
Examples of SP³ Hybridized Molecules
1. Methane (CH₄)
- Central atom: Carbon (sp³ hybridized)
- Geometry: Tetrahedral
- Bond angle: 109.5°
2. Ethane (C₂H₆)
- Each carbon is sp³ hybridized, forming C-C and C-H σ bonds.
- Free rotation around single bonds (unlike sp² double bonds).
3. Ammonia (NH₃) and Water (H₂O)
- NH₃: sp³ hybridization with one lone pair (pyramidal shape).
- H₂O: sp³ hybridization with two lone pairs (bent shape).
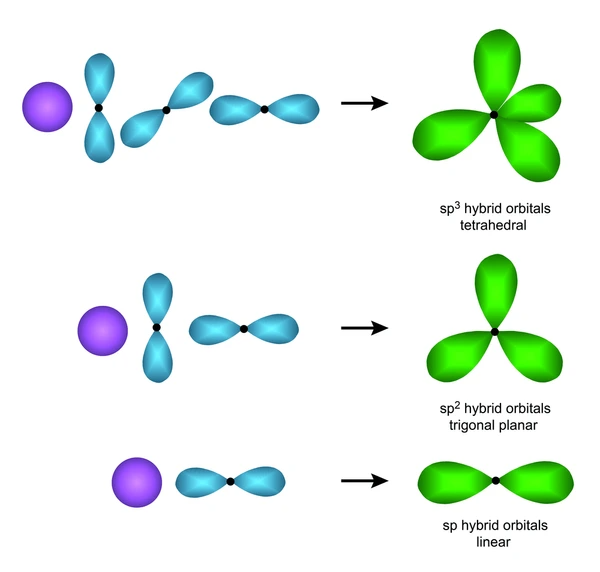
Comparison with SP² and SP Hybridization
SP² hybridization involves the mixing of one s orbital and two p orbitals, resulting in three equivalent SP² hybrid orbitals. These orbitals orient themselves at 120 degrees to each other and participate in sigma (σ) bonding. Molecules like benzene (C₆H₆) and ethylene (C₂H₄) commonly exhibit SP² hybridization in their trigonal planar geometry.
On the other hand, SP hybridization involves the mixing of one s orbital and one p orbital, resulting in two equivalent SP hybrid orbitals. These orbitals orient themselves at 180 degrees to each other and form sigma (σ) bonds. Molecules like carbon dioxide (CO₂) and acetylene (C₂H₂) commonly exhibit SP hybridization in their linear geometry.
The main differences between SP² and SP hybridization lie in the number of orbitals involved and the geometry of the molecules they form. SP² hybridization results in three equivalent orbitals with a trigonal planar geometry, while SP hybridization results in two equivalent orbitals with a linear geometry. Additionally, SP² hybridization is associated with sp² mixing, while SP hybridization is associated with sp mixing.
| Hybridization | Orbitals Mixed | Geometry | Bond Angle | Example |
|---|---|---|---|---|
| sp³ | 1s + 3p | Tetrahedral | 109.5° | CH₄ |
| sp² | 1s + 2p | Trigonal Planar | 120° | C₂H₄ (Ethene) |
| sp | 1s + 1p | Linear | 180° | C₂H₂ (Ethyne) |
- sp² (double bonds) and sp (triple bonds) have higher p-character and different geometries.
Bonding Properties
1. Single Bond Flexibility
- σ bonds in sp³ hybridization allow free rotation (e.g., alkanes).
- Explains conformational isomerism in organic molecules.
2. Reactivity of sp³ Carbon
- Nucleophilic substitution (SN1/SN2) in alkyl halides.
- Radical reactions (e.g., halogenation of alkanes).
3. Biological Importance
- Tetrahedral carbon is the backbone of biomolecules (DNA, proteins, lipids).
- Enzyme active sites often involve sp³ hybridized atoms.
Application Cases
| Product/Project | Technical Outcomes | Application Scenarios |
|---|---|---|
| Metal-Organic Framework Devices Sony Group Corp. | Regulates structure with transition metal ions and heterocyclic ligands to enable stable and functional molecular devices | Technological applications requiring stable molecular devices inspired by enzyme principles |
| 3D Graphene Microlattice Texas Instruments Incorporated | Produces uniform sp2-bonded carbon structures using photopolymer waveguide technique | Applications requiring strong and conductive graphene microstructures |
| Gradia Carbon Yanshan University | Novel sp2-sp3 hybrid crystalline carbon synthesized under high temperature and pressure | Applications requiring tunable carbon allotropes with varying sp2 and sp3 structural unit ratios |
| Strengthened PAN-based Carbon Fibers Jeonbuk National University | Increases carbon sp3 bonding and nitrogen atoms with quaternary bonding using slow heating rate carbonization | Applications requiring high-strength carbon fibers with improved mechanical performance |
| Carbon-based Nanomaterial Sensors University of Trento | Utilizes unique properties of carbon nanomaterials for enhanced sensing capabilities | Biocompatible sensing applications requiring high sensitivity, selectivity, and low detection limits |
Conclusion
sp³ hybridization is essential for understanding tetrahedral molecular geometry, bond angles, and the behavior of single-bonded carbon compounds. From methane to complex biomolecules, this concept underpins much of organic chemistry.By mastering sp³ hybridization, chemists can predict molecular shapes, reactivity, and even design new drugs and materials.
To get detailed scientific explanations of sp³ Hybrid Orbitals, try Patsnap Eureka.


