
As the fundamental reference electrode in electrochemistry, the Standard Hydrogen Electrode (SHE) establishes the universal baseline for measuring electrode potentials. Chemists assign it a potential of 0.00 volts under standard conditions, making it indispensable for determining standard reduction potentials of all electrochemical half-reactions. This critical reference plays a vital role across scientific and industrial applications, from battery development to corrosion studies and pH measurement systems.
The SHE operates through hydrogen ion reduction, where gaseous hydrogen (H₂) at standard pressure (1 atm) interacts with hydrogen ions (H⁺) in solution under precisely controlled conditions (1 M concentration at 25°C/298 K). While conceptually simple, researchers must carefully maintain experimental conditions to ensure the electrode’s reliability and accuracy in electrochemical research.
Definition and Working Principle
What makes the Standard Hydrogen Electrode (SHE) the gold reference in redox reactions? Eureka Q&A demystifies SHE’s setup, function, and pivotal role in measuring electrode potentials—making complex concepts crystal clear for students and researchers alike.
The Standard Hydrogen Electrode functions as a reversible redox electrode with the half-cell reaction:
2H⁺(aq) + 2e⁻ ⇌ H₂(g)
The international scientific community has established this reaction’s electrode potential as 0.00 V under standard conditions (1 M H⁺, 1 atm H₂, 25°C). Platinum’s unique properties make it ideal for SHE construction – when coated with platinum black, it provides both the necessary surface area and catalytic activity for efficient electron transfer between H₂ gas and H⁺ ions. Importantly, platinum’s inert nature prevents electrode participation in the reaction, ensuring stable and reproducible reference potential measurements.
- Reference Point: The SHE serves as a universal reference point for measuring the potential of other electrodes. All other electrode potentials are relative to the SHE.
- Thermodynamic Scale: It allows for the establishment of a thermodynamic scale of electrode potentials, which is essential for comparing the reduction potentials of different redox couples.
- Calibration: Other reference electrodes, such as the Reversible Hydrogen Electrode (RHE), are often calibrated against the SHE to ensure accurate potential measurements.
- Electrochemical Measurements: The SHE is used in various electrochemical measurements and experiments to provide a stable and reliable reference point.
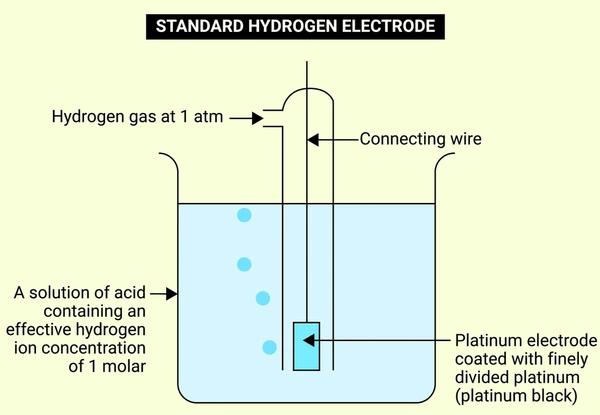
Construction and Setup
Building a functional Standard Hydrogen Electrode requires several precise components:
- Platinum Electrode: High-purity platinum wire or foil, typically platinized to enhance reaction kinetics
- Gas Delivery System: Continuous ultra-pure H₂ gas flow at 1 atmosphere pressure
- Acidic Electrolyte: HCl or H₂SO₄ solution maintaining exact 1 M H⁺ concentration
- Temperature Regulation: Precise maintenance at 25°C (298 K)
- Connection System: Porous diaphragm or KCl salt bridge for half-cell connection
After assembly, technicians immerse the electrode in the acidic solution while hydrogen gas flows across the platinum surface. This configuration establishes the crucial redox equilibrium that serves as the reference point for all electrochemical measurements.
Key Applications in Electrochemistry
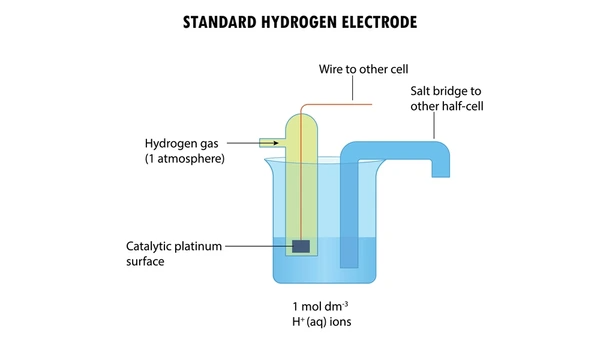
The SHE serves multiple essential functions in electrochemical research and applications:
Reference Standardization
Scientists measure all standard reduction potentials (E°) relative to SHE. For instance, a zinc electrode showing -0.76 V versus SHE indicates greater reducing power than hydrogen, while a copper electrode at +0.34 V demonstrates stronger oxidizing capability.
Reaction Spontaneity Determination
By comparing electrode potentials against SHE, researchers can predict redox reaction spontaneity. Any cell potential (E°cell) greater than 0 confirms a thermodynamically favorable reaction.
pH Measurement Foundation
The SHE’s fundamental relationship with hydrogen ion concentration underpins modern pH measurement systems through the Nernst equation.
Industrial and Research Uses
- Battery and fuel cell development – electrode material evaluation
- Corrosion science – metal electrochemical behavior analysis
- Bioelectrochemistry – enzyme redox potential studies
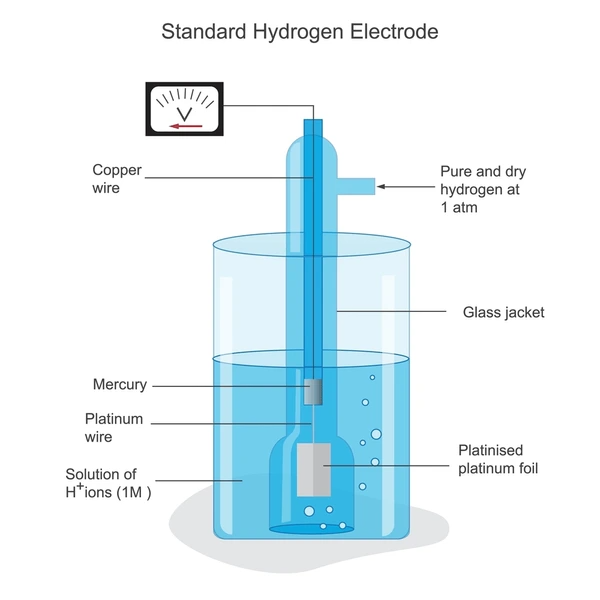
Application Cases
| Product/Project | Technical Outcomes | Application Scenarios |
|---|---|---|
| Phase Inversion Electrode Synthesis Massachusetts Institute of Technology | Tunable microstructures for porous carbon electrodes, improving performance and reducing costs | Redox flow batteries and other electrochemical systems |
| Conductive Diamond Electrode De Nora Permelec Ltd. | Enhanced durability and corrosion resistance through fluorination treatment | Water treatment and industrial electrolysis processes |
| Electrode-Membrane Assembly De Nora Permelec Ltd. | Improved electrical conductivity and mechanical strength through thermoplastic resin penetration | Fuel cells and electrolytic cells |
| 2D Electrochemical Surface Potential Control Corning, Inc. | Efficient generation and detection of candidate materials through non-linearly varying electric fields | Combinatorial experiments and rapid substance detection |
| Layered Diamond Electrode Element Six Ltd. | Improved durability and performance with heavily doped core and lightly doped surface | Electrochemical processing requiring high durability and efficiency |
Practical Considerations
Advantages
- Universal acceptance as primary electrochemical reference
- Simple, well-understood underlying redox chemistry
- Excellent reproducibility under controlled conditions
Limitations
- Challenging maintenance requirements
- Expensive platinum components with limited lifespan
- Impractical for routine laboratory use
Practical Alternatives
Most laboratories prefer these SHE-calibrated alternatives for daily use:
- Silver/Silver Chloride (Ag/AgCl): Common in pH meters (+0.197 V vs SHE)
- Saturated Calomel Electrode (SCE): Mercury-based (+0.241 V vs SHE)
- Copper-Copper Sulfate (CSE): Specialized for corrosion monitoring
Conclusion
As the gold standard in electrochemistry, the Standard Hydrogen Electrode continues to provide the essential reference framework for electrode potential measurements. Despite its practical challenges in routine use, SHE remains theoretically irreplaceable. Modern laboratories typically employ secondary reference electrodes like Ag/AgCl or SCE, but all ultimately trace their calibration back to SHE. For researchers in battery technology, corrosion science, and analytical chemistry, mastering SHE principles remains fundamental to electrochemical understanding and innovation.
FAQs
A1: SHE provides a stable, reproducible 0.00V baseline for comparing all other electrode potentials.
A2: SHE requires stringent conditions (1atm H₂, 1M H⁺, 25°C) that are difficult to maintain routinely.
A3: Ag/AgCl and SCE electrodes are preferred alternatives as they’re easier to use while being SHE-calibrated.
A4: SHE’s potential depends on H⁺ concentration, forming the theoretical basis for pH measurements.
A5: Yes, when testing stronger reducing agents like zinc (-0.76V vs SHE).
To get detailed scientific explanations of standard hydrogen electrodes, try Patsnap Eureka.


