
Surface Plasmon Resonance (SPR) is a powerful optical technique used to detect molecular interactions in real time without labeling. Widely used in biochemistry, pharmaceuticals, and materials science, SPR helps scientists analyze binding kinetics, affinity, and concentration changes at the molecular level. Its non-invasive nature and high sensitivity make it an essential tool in modern biosensing and diagnostics.
This article explores the working principle of surface plasmon resonance, its key applications, and the benefits it brings to research and development.
What Is Surface Plasmon Resonance?
What is Surface Plasmon Resonance (SPR)? Eureka Technical Q&A explains that SPR is a powerful optical technique used to detect molecular interactions in real time, widely applied in biosensing, drug discovery, and biochemical research.
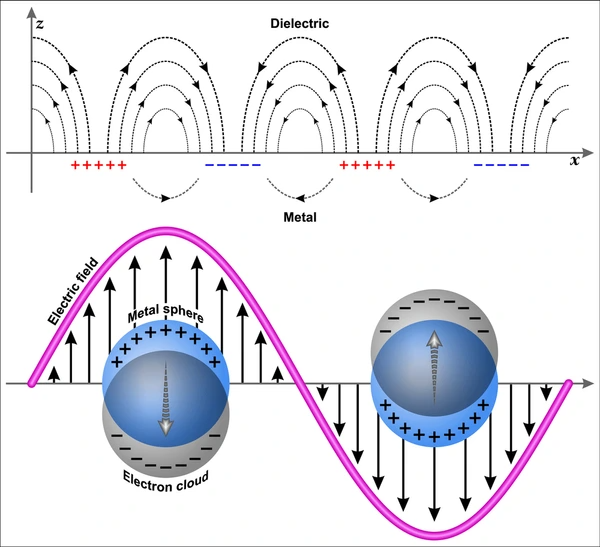
Surface Plasmon Resonance is a physical phenomenon that occurs when polarized light hits a thin metal film—typically gold—at a specific angle, causing free electrons on the metal surface to oscillate collectively. These oscillations, known as surface plasmons, are sensitive to changes at the metal-dielectric interface.
When molecules bind to the metal surface, they alter the refractive index near the surface. This change shifts the angle at which SPR occurs, allowing researchers to detect and measure the interaction in real time.
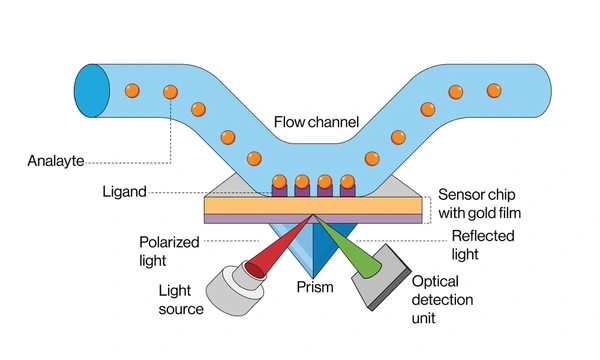
How SPR Works
- Researchers prepare a sensor chip by coating it with a thin gold film and adding a functional layer to immobilize biomolecules like proteins or DNA.
- Polarized light passes through a prism and reflects off the back of the metal film at varying angles.
- At a specific angle—called the resonance angle—the system transfers energy from the light to surface plasmons, causing a dip in reflected light intensity.
- When target molecules bind to the immobilized layer, the local refractive index changes, shifting the resonance angle.
- The system monitors and records this shift, generating a real-time sensorgram that reflects molecular interactions.
SPR does not require fluorescent or radioactive labeling, making it ideal for studying biological systems in their native state.
Key Parameters Measured by SPR
- Association rate constant (ka)
Measures how quickly two molecules bind - Dissociation rate constant (kd)
Indicates how quickly the complex falls apart - Equilibrium dissociation constant (KD)
Reflects binding affinity; a lower KD means stronger binding - Concentration
Determines the amount of analyte in a sample based on binding response
Applications of Surface Plasmon Resonance
Drug Discovery and Development
Pharmaceutical researchers widely use SPR to screen drug candidates and measure how well they bind to biological targets such as enzymes, receptors, or antibodies. It supports structure-activity relationship (SAR) analysis and helps in hit-to-lead optimization.
Biomolecular Interaction Analysis
Scientists use SPR to investigate protein-protein, protein-DNA, protein-lipid, and antibody-antigen interactions. This information is crucial for understanding cellular signaling pathways and disease mechanisms.
Clinical Diagnostics
SPR-based biosensors can detect biomarkers for diseases such as cancer, COVID-19, or cardiovascular conditions. These systems enable fast, label-free detection in blood, saliva, or urine samples.
Food Safety and Environmental Monitoring
SPR technology is applied to detect contaminants, toxins, and pathogens in food and water. Its sensitivity allows for early identification of harmful substances at very low concentrations.
Material Science and Nanotechnology
Researchers use SPR to study surface modifications, thin film coatings, and nanoparticle interactions. It plays a role in sensor development, surface chemistry, and the design of smart materials.
Application Cases
| Product/Project | Technical Outcomes | Application Scenarios |
|---|---|---|
| Enhanced SPR Sensor Fraunhofer-Gesellschaft eV | Improved sensitivity through nanodiamonds embedded in gold layer, increasing plasmon wave coupling and scattering | More accurate detection of target molecules in biological and chemical sensing applications |
| SPR Cancer Diagnostic Tool Nankai University | Effective detection of ultra-low concentration biomarkers | Early diagnosis of cancer through non-invasive biomarker detection |
| Microfluidic SPR Biosensor Stanford University | Integration of microfluidics with SPR for improved sample handling and analysis | Continuous transport, cross transport, and fluid dynamic transport designs for various biosensing applications |
| Point-of-Care SPR Diagnostic Tool CSIR South Africa | Real-time monitoring of molecular binding for biological and chemical sensing | Potential development of portable diagnostic devices for on-site testing |
| Fiber Optic SPR Sensor Indian Institute of Technology New Delhi | Enhanced sensitivity using nanomaterials and diverse geometrical designs | Chemical and biological entity detection in environmental monitoring and clinical diagnostics |
Benefits of Using SPR
- Label-Free Detection
No need for fluorescent or radioactive tags, preserving molecular integrity - Real-Time Monitoring
Allows dynamic measurement of binding events as they happen - High Sensitivity
Detects interactions with analyte concentrations in the picomolar to nanomolar range - Versatile Analyte Range
Suitable for small molecules, proteins, nucleic acids, lipids, and complex biomolecules - Reusable Sensor Chips
Some chips can be regenerated and reused, lowering experimental costs
Limitations to Consider
While SPR offers numerous advantages, it does have limitations:
- Surface Immobilization Required
One binding partner must be attached to the sensor chip, which may alter behavior - Sensitive to Temperature and Refractive Index
Environmental changes can affect readings - Not Ideal for Complex Mixtures Without Separation
Sample purity may influence data accuracy
FAQs
Is SPR only used for biological molecules?
No. While commonly used in biochemistry, SPR also applies to chemical sensors, environmental monitoring, and materials science.
Can SPR measure the strength of binding?
Yes. It determines binding affinity through equilibrium dissociation constants (KD), giving insight into how strong a molecular interaction is.
What kind of surfaces are used in SPR?
SPR typically uses gold-coated glass chips, as gold supports surface plasmon waves and is biocompatible.
Is SPR the same as localized SPR (LSPR)?
No. SPR measures wave propagation along a flat metal surface, while LSPR involves nanoparticles and offers higher sensitivity in small volumes.
How long does a typical SPR experiment take?
Researchers can complete most binding studies within minutes to a few hours, depending on the interaction’s complexity.
Conclusion
Surface Plasmon Resonance is a vital analytical tool that allows scientists to observe molecular interactions in real time with high precision. From drug discovery to disease diagnostics and materials engineering, SPR continues to transform how we analyze biological and chemical systems. Its combination of label-free detection, sensitivity, and versatility makes it an indispensable technique in cutting-edge research and industry applications.
To get detailed scientific explanations of Surface Plasmon Resonance, try Patsnap Eureka.


