
In organic chemistry, many molecules can exist in more than one structural form without breaking their bonds completely. This dynamic interconversion is known as tautomerization, a reversible process that plays a crucial role in biological systems, drug behavior, and chemical reactions.
One of the most common examples is the keto-enol tautomerism, but it’s not the only type. In this article, you’ll learn what tautomerization is, how it works, the major types, and where it’s found in real-world chemistry.
What Is Tautomerization?
Need help visualizing how tautomerization works or when it matters in organic reactions? Eureka Technical Q&A connects you with real chemistry experts who offer clear, fast explanations—making it easier to understand reaction mechanisms, resonance structures, and their impact on chemical behavior.
Tautomerization is a chemical reaction in which one structural form of a compound (a tautomer) reversibly converts into another by the relocation of a hydrogen atom and a double bond. These forms are called tautomers and exist in dynamic equilibrium. Tautomerization is a type of isomerism that involves the migration of a hydrogen atom accompanied by changes in valency. This process is often seen in compounds with functional groups like keto and enol, amide and imide, lactam and lactim, and enamine and imine. For example, in the keto-enol tautomerization, a keto group can convert to an enol group, and vice versa.
Unlike resonance structures, which involve delocalized electrons, tautomers are distinct compounds with different connectivity of atoms.
Keto-Enol Tautomerism: The Most Common Example

Basic Concept
In keto-enol tautomerism, a ketone (or aldehyde) form interconverts with its corresponding enol form. The enol has a hydroxyl group (-OH) bonded to a carbon-carbon double bond.
General Equation:
Keto form: R–C(=O)–CH₂– → ← HO–C=CH– Enol form
Key Points:
- The keto form is usually more stable due to stronger C=O bonding.
- The enol form becomes relevant in acidic or basic conditions or when it stabilizes through hydrogen bonding or aromaticity.
Example: Acetone
CH₃–CO–CH₃ ⇌ CH₂=CH(OH)CH₃
Though the keto form dominates, the enol exists in trace amounts and can influence reactivity in enolate chemistry.
Types of Tautomerism
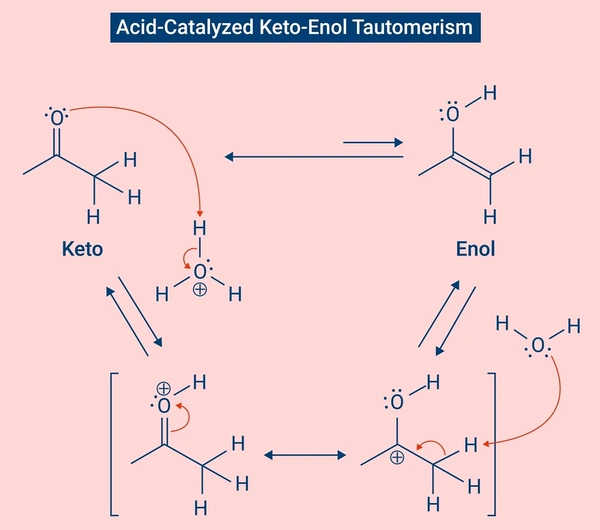
1. Keto–Enol Tautomerism
Occurs in carbonyl compounds. Common in aldehydes, ketones, and β-diketones.
Importance: Central to enolate chemistry and aldol reactions.
2. Enamine–Iminium Tautomerism
Involves amines and imines. Tautomerism occurs between an enamine (double bond next to a nitrogen) and an iminium ion.
Example: In pyrrolidine-catalyzed reactions.
3. Lactam–Lactim Tautomerism
Found in cyclic amides (lactams), particularly in heterocyclic systems like purines and pyrimidines.
Example: Uracil and thymine in DNA undergo lactam-lactim tautomerization.
4. Amide–Imidic Acid Tautomerism
Tautomerism between amide and imidic acid forms. Less common but appears in hydrolysis or catalysis steps.
5. Nitroso–Oxime Tautomerism
Occurs between nitroso (R–N=O) and oxime (R–C=NOH) structures. Important in dye chemistry and analytical methods.
Mechanism of Tautomerization
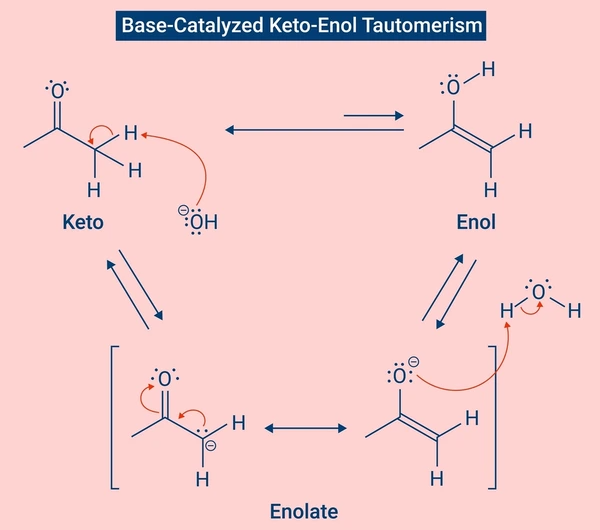
The mechanism depends on the type of tautomerism and the reaction conditions.
Acid-Catalyzed Tautomerism (Keto-Enol):
- Protonation of the carbonyl oxygen
- Formation of a carbocation
- Rearrangement of the hydrogen to form the enol
Base-Catalyzed Tautomerism:
- Abstraction of α-hydrogen to form an enolate ion
- Protonation of oxygen to form the enol
Biological Relevance
Tautomerization is not just a lab phenomenon—it affects life at the molecular level.
- DNA base pairing: Rare tautomeric forms of adenine or thymine can lead to mutations during replication.
- Drug design: Tautomers may bind differently to biological receptors, impacting drug efficacy and selectivity.
- Enzyme catalysis: Many enzymes rely on keto-enol tautomerization during the reaction cycle.
How to Identify Tautomers
- Understanding Tautomerism:
- Tautomers are structural isomers that exist in a dynamic equilibrium, readily convertible into each other through proton shifts or electron migrations. Common types include keto-enol tautomerism and imine-enamine tautomerism.
- Theoretical Methods:
- Use tautomerization calculators and algorithms to identify tautomeric regions within a molecule. These tools can enumerate all possible tautomers and predict their distribution based on factors like pKa and temperature.
- Implement ChemAxon’s tautomerization plugin, which identifies proton donors and acceptors, and computes tautomerization paths to generate all tautomeric forms.
- Experimental Techniques:
- Employ chromatography techniques to separate and analyze tautomeric mixtures. Advances in chromatography have enabled the separation of atropic isomers in certain cases.
- Utilize crystallographic characterization to identify tautomers by determining the localized systems of π-bonding and confirming the structural isomers experimentally.
- Software and Tools:
- Leverage cheminformatics tools that offer tautomer duplicate search and substructure search functionalities to handle tautomerism efficiently.
- Use standardization tools integrated with chemical database systems to perform custom transformations and handle ring-chain tautomerism.
- Considerations for Accuracy:
- Ensure that all chemically reasonable tautomers are identified to avoid missing potential isomers. This involves considering various factors like temperature, solvent, and pH that influence the tautomeric equilibrium.
- Validate the identified tautomers through experimental methods like crystallography to confirm their structures and stability.
FAQs
Tautomerism involves actual chemical equilibrium between two compounds with different atomic connectivities. Resonance involves electron delocalization within a single compound.
Sometimes. If one form is much more stable, the other may only exist in trace amounts. But in some cases (e.g., β-diketones), both can be observed.
Not all, but those with α-hydrogens are capable of undergoing keto-enol tautomerism.
The carbonyl group has a strong C=O double bond, which is energetically favorable compared to a C=C bond in the enol.
Yes, it’s a dynamic equilibrium process that can shift depending on pH, temperature, and solvent.
Conclusion
Tautomerization is a fascinating and essential concept in chemistry, where molecules shift between distinct structures by relocating atoms and bonds. Among the different types, keto-enol tautomerism is the most commonly encountered and has major implications in organic synthesis, biochemistry, and pharmaceuticals.
By understanding tautomerization, chemists can predict molecular behavior, control reaction mechanisms, and appreciate how even small shifts in structure can lead to major changes in function.
To get detailed scientific explanations of Tautomerization, try Patsnap Eureka.


