Introduction
Thermite, renowned for its blazing heat and remarkable applications, has captured the imagination of scientists and engineers alike. This remarkable substance has the ability to generate intense heat, making it a powerful tool in various industries.
In this comprehensive guide, we delve into the fascinating world of thermite, exploring its origins, scientific principles, and diverse applications. We also examine its pros and cons, highlighting its current uses and discussing its potential for future innovation.
What is Thermite?
Thermite is a pyrotechnic composition consisting of a metal powder fuel, typically aluminum, and a metal oxide, commonly iron oxide (Fe₂O₃). Known for its ability to generate intense heat upon ignition, it undergoes a highly exothermic reduction-oxidation (redox) reaction, sometimes reaching temperatures up to 3000°C.

Basic Composition and Reactions
The key components of thermite are a metal fuel like aluminum (Al), magnesium (Mg), or silicon (Si), and a metal oxide oxidizer such as iron(III) oxide (Fe₂O₃), copper(II) oxide (CuO), or manganese(IV) oxide (MnO₂). For example, a common thermite composition includes aluminum and iron(III) oxide:
2Al+Fe2O3→2Fe+Al2O3
Upon ignition by an external heat source such as a spark or flame, the metal fuel reduces the metal oxide, producing molten metal and aluminum oxide. This reaction is highly exothermic, generating temperatures up to 2500°C or higher. Thermite reactions are self-sustaining and produce significant heat, light, and sparks, yielding molten metal and solid metal oxide as products. These reactions are relatively insensitive to external stimuli, making them safer to handle compared to conventional explosives.
Historical Background of Thermite
Discovery and Early Use
- Accidental Discovery: Thermite was discovered in 1893 by chemists at Goldschmidt AG while developing incendiary materials. They found that aluminum metal and iron oxide reacted vigorously when ignited.
- Formalization: German chemist Hans Goldschmidt formalized the discovery in the late 19th century.
- Initial Applications: Initially used for metal refining without carbon, thermite’s high-temperature reactions made it suitable for welding and cutting metals in industrial applications.

Evolution of Applications
- Early Welding: Thermite was adopted for welding, especially for joining rails in the railroad industry.
- Military Uses: During the World Wars, thermite was used in incendiary bombs, grenades, and demolition charges.
- Manhattan Project: Yes, thermite played a role in uranium purification for the Manhattan Project.
- Nanothermites: In the early 2000s, nanothermites with nanometer-sized particles were developed, offering higher reaction rates and increased sensitivity.
- Current Applications: Nanothermites opened new applications in microinitiators, microdetonators, self-destructing systems, pyrotechnics, solid rocket propellants, and microfabrication processes.
To know more about the latest research and development achievement of thermite in different industries, you can ask Eurkea.
Scientific Explanation of Thermite Reactions
Chemical Principles
The thermite reaction, a type of reduction-oxidation (redox) process, occurs when a reactive metal such as aluminum (Al) reduces a metal oxide like iron oxide (Fe2O3), yielding molten iron (Fe) and aluminum oxide (Al2O3). The reaction is represented by the equation:
Fe2O3+2Al→2Fe+Al2O3Fe2O3+2Al→2Fe+Al2O3
In this exothermic reaction, aluminum acts as the reducing agent, removing oxygen from the iron oxide, resulting in aluminum being oxidized to aluminum oxide while iron oxide is reduced to elemental iron.
Energy and Temperature
Thermite reactions are highly exothermic, releasing substantial heat and requiring no external heat source to sustain. Temperatures in these reactions can exceed 2500°C (4500°F), making thermite useful for applications like welding and metallurgy due to its ability to generate high heat and melt metals like iron.
In essence, thermite reactions involve a reactive metal like aluminum, which serves as a powerful reducing agent in these high-temperature, exothermic processes, beneficial for various industrial uses.

Pros and Cons of Thermite
Pros
- High Temperature and Energy Output: Thermite reactions achieve temperatures exceeding 2500°C (4500°F), ideal for high-heat applications like welding and metal cutting.
- Efficiency in Welding: The ability to weld large metal structures efficiently due to its intense heat and energy release.
- Controlled Industrial Use: Once ignited, the reaction is self-sustaining and can be precisely managed in controlled settings.
Cons
- Safety Hazards: The extreme temperatures and reactive nature of thermite pose significant safety risks if not handled correctly.
- Limited Applications: The specific high-heat conditions required for thermite reactions restrict its use to particular environments that can support such conditions.
- Environmental and Health Concerns: Some metal oxides used in thermite can be toxic, requiring careful handling and disposal to mitigate environmental impact.
Common and Industrial Applications of Thermite
Interested in the applications of thermite? Eureka Technical Q&A provides in-depth insights into its use in metal cutting, welding, and military applications, helping you explore its industrial significance and safety considerations for various sectors.
Welding
Thermite is extensively used in the welding of large iron or steel sections, particularly in the construction and maintenance of railroads. The high temperatures generated by thermite reactions enable the welding of thick, heavy sections efficiently and effectively, ensuring strong, durable joints.
Metal Purification
In addition to its welding applications, thermite is occasionally utilized in the purification of metal ores. This process is particularly notable in the extraction of uranium, where thermite reactions help in reducing and purifying the metal from its ores, enhancing the quality and purity of the extracted metal.
BiSN’s Use of Thermite
BiSN Technologies leverages thermite in innovative ways, particularly in oil and gas well completions. They utilize controlled thermite heaters to melt bismuth alloy for downhole sealing applications, ensuring robust and leak-proof seals. Additionally, BiSN develops custom thermite formulations—termed as thermite crumbles—designed to perform optimally under varying environmental conditions and specific application needs, demonstrating the versatility and adaptability of thermite in industrial applications.
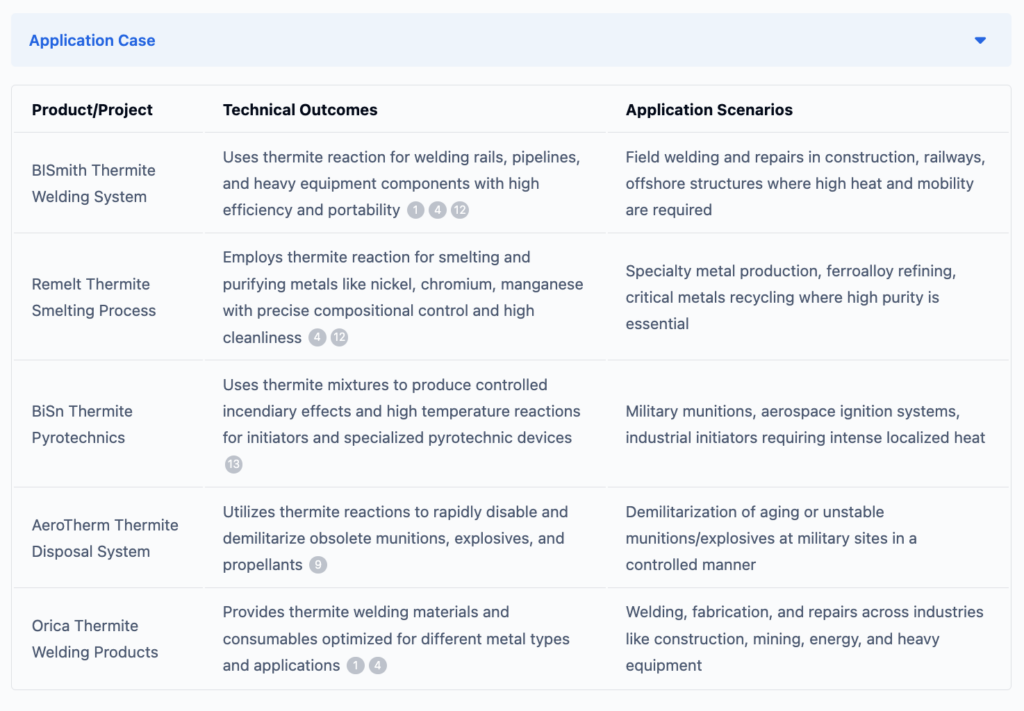
If you want to know more about applications of thermite, to help improve your research and development projects or innovations, ask Eureka Tech-Seek.
Safety and Handling of Thermite
Safe Usage Practices
- Controlled Environments: Essential for minimizing safety risks during thermite usage.
- Safety Protocols: Companies like BiSN enforce strict guidelines and training to ensure safe handling.
Potential Risks
- Uncontrolled Reactions: High temperatures and potential for explosive reactions require careful management.
- Storage and Ignition: Proper storage away from ignition sources and careful handling are crucial to prevent accidents.
- Containment and Ventilation: Necessary to manage heat and fumes during reactions, ensuring a safe working environment.
While thermite reactions offer valuable applications, their high energy output and extreme temperatures necessitate stringent safety measures and cautious handling procedures to mitigate potential hazards.

Future Potential of Thermite
Technological Innovations
- Research and Development: Continuous advancements in thermite technology are exploring more efficient and varied applications across different industries.
- Material Synthesis: The intense heat from thermite is being used to synthesize advanced materials like ceramics and carbides.
Emerging Applications
- Downhole Operations: BiSN is pioneering the use of liquid thermite for heating in oil and gas extraction, potentially eliminating the need for section milling.
- Well Plugging: Use of thermite for setting permanent plugs in challenging well architectures.
Environmental and Industrial Implications
- Sustainability: The self-sustaining nature of thermite reactions offers environmentally friendly alternatives for high-energy processes.
- Gas Generation: Thermite’s ability to generate gases like hydrogen on-demand presents new opportunities for clean energy applications.
For detailed scientific explanations and in-depth research of innovations of thermite, you can always get what you want in Eureka Technical Research.

Conclusion
Thermite, characterized by its high-temperature reactions and versatile applications, has evolved significantly from its initial discovery to modern industrial uses. This comprehensive guide has explored thermite’s chemical properties, historical significance, current applications, and future potential. By harnessing thermite’s capabilities, industries continue to innovate, particularly in welding, metal purification, and even energy production, promising further advancements and safety enhancements in its application.

