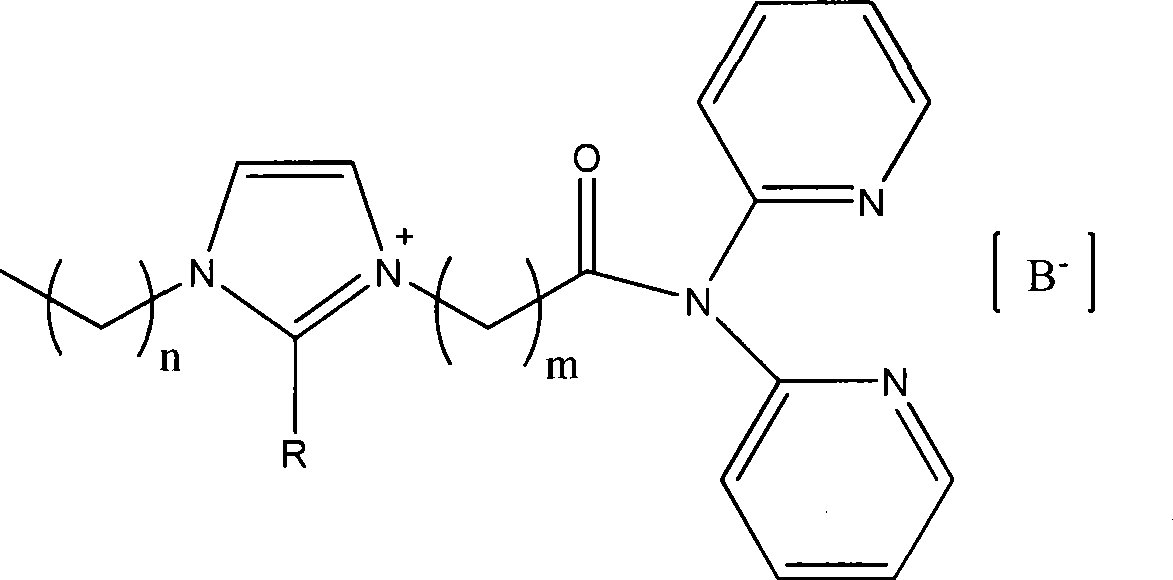
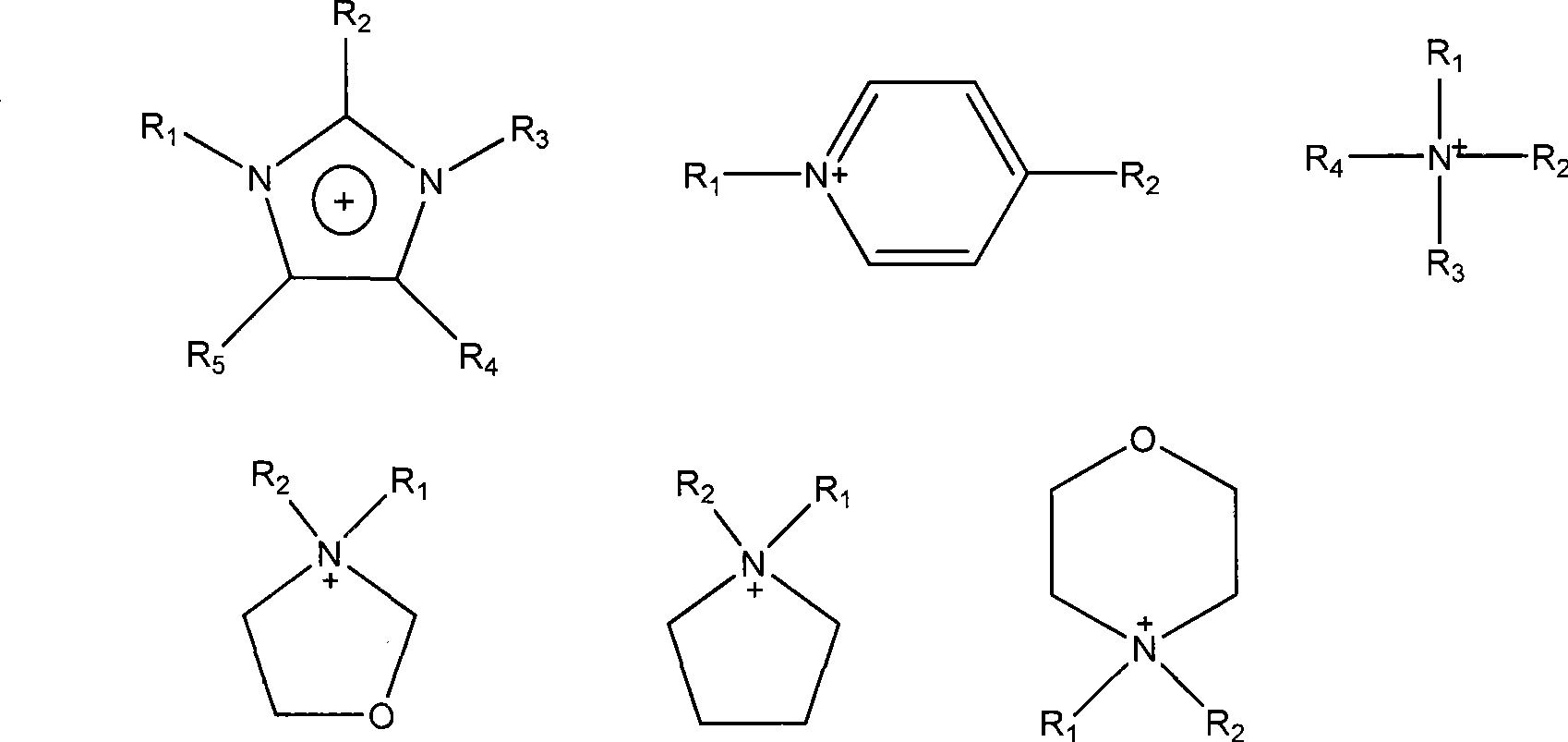
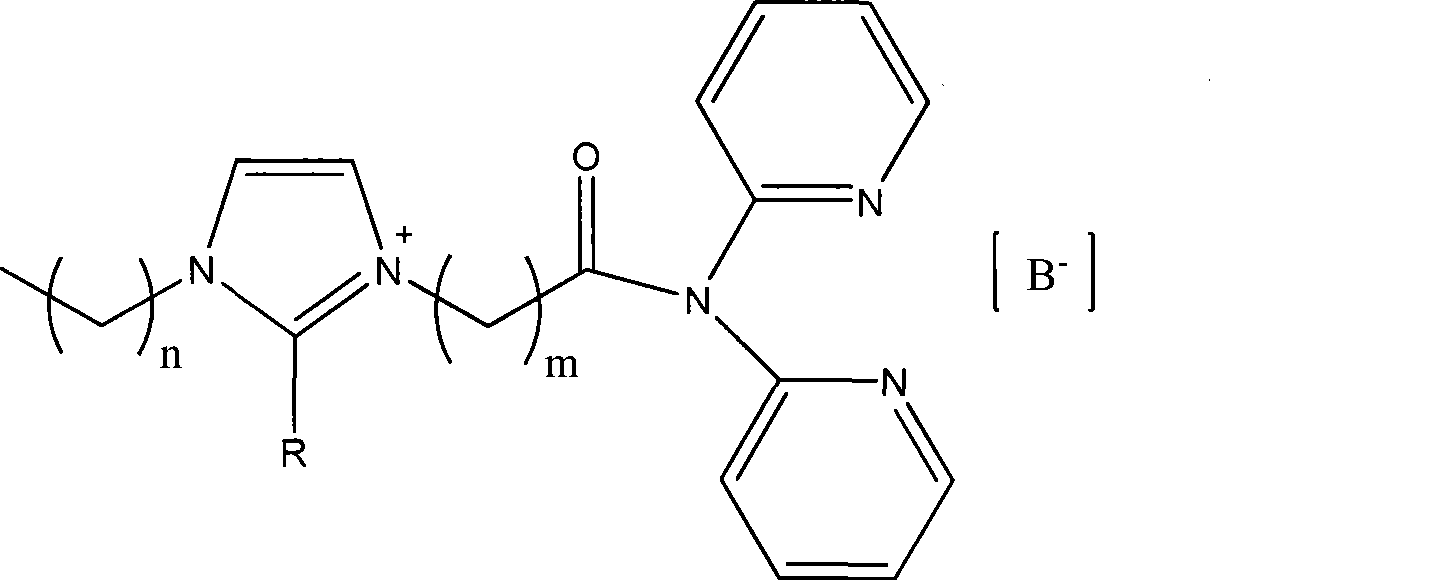
Functional ino liquid containing bidentate nitrogen ligands and synthesis method thereof
A technology of ionic liquid and bidentate nitrogen, which is applied in the field of new materials and its preparation, achieves the effects of high yield, simple synthesis steps, and reduced loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Under a nitrogen atmosphere, add 2.92 mmol of 2,2'-dipyridylamine and equimolar triethylamine into a three-necked flask containing 15 ml of dichloromethane, cool to -20°C in an ice-salt bath, and then gradually 11.68 mmol of 3-chloropropionyl chloride was added dropwise, the temperature was gradually raised to room temperature, and stirred overnight. Transfer to a 60ml separatory funnel, add 8ml of cooled saturated sodium carbonate solution, wash with water (2×4ml) twice, separate the organic phase, dry over sodium sulfate, filter, and drain the solvent under reduced pressure to obtain 3-chloro-N,N - Bis(2-pyridine)propionamide. Synthetic chemical formula:
[0032]
[0033] (2) Under a nitrogen atmosphere, 2.56 mmol of 3-chloro-N, N-di(2-pyridine) propionamide and 2.81 mmol of 1-methylimidazole were dissolved in 15 ml of acetonitrile, refluxed for 8 hours, and the acetonitrile was drained, and then Dissolve in 20ml of deionized water, wash with toluene (3×5ml),...
Embodiment 2
[0036] (1) Under a nitrogen atmosphere, add 2.92 mmol of 2,2'-dipyridylamine and equimolar triethylamine into a three-necked flask containing 15 ml of dichloromethane, cool to -20°C in an ice-salt bath, and then gradually 11.68 mmol of 3-chloropropionyl chloride was added dropwise, the temperature was gradually raised to room temperature, and stirred overnight. Transfer to a 60ml separatory funnel, add 8ml of cooled saturated sodium carbonate solution, wash with water (2×4ml) twice, separate the organic phase, dry over sodium sulfate, filter, and drain the solvent under reduced pressure to obtain 3-chloro-N,N - Bis(2-pyridine)propionamide. Synthesis reaction formula:
[0037]
[0038] (2) Under a nitrogen atmosphere, dissolve 2.56mmol of 3-chloro-N,N-bis(2-pyridine)propionamide and 2.81mmol of 1,2-dimethylimidazole in 15ml of acetonitrile, reflux for 8 hours, and drain Acetonitrile was redissolved in 20ml deionized water, washed with toluene (3×5ml), and the solvent was d...
Embodiment 3
[0041] (1) Under a nitrogen atmosphere, add 2.92 mmol of 2,2'-dipyridylamine and equimolar triethylamine into a three-necked flask containing 15 ml of dichloromethane, cool to -20°C in an ice-salt bath, and then gradually 11.68 mmol of 3-chloropropionyl chloride was added dropwise, the temperature was gradually raised to room temperature, and stirred overnight. Transfer to a 60ml separatory funnel, add 8ml of cooled saturated sodium carbonate solution, wash with water (2×4ml) twice, separate the organic phase, dry over sodium sulfate, filter, and drain the solvent under reduced pressure to obtain 3-chloro-N,N - Bis(2-pyridine)propionamide. Synthesis reaction formula:
[0042]
[0043] (2) Under a nitrogen atmosphere, dissolve 1-ethylimidazole containing 2.56mmol and 2.81mmol of bidentate nitrogen ligand 3-chloroamide in 1 in 15ml of acetonitrile, reflux for 8 hours, drain the acetonitrile, and then dissolve in 20ml Wash with deionized water and toluene (3×5ml), and dry th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com