Method for preparing tributyl citrate by using rare-earth salt binary complex type solid acid as catalyst
A technology catalyzed by tributyl citrate and solid acid, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of corrosion equipment, complicated preparation process, and complicated post-treatment, and achieve The effects of no corrosion to equipment, small dosage, and cheap catalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0021] Example 1
[0022] Step 1 Preparation of compound catalyst
[0023] Weigh 0.63 g of cerium sulfate tetrahydrate and sulfamic acid in a weight ratio of 2:1, and mix them evenly to obtain a binary compound catalyst.
[0024] Step 2 esterification reaction
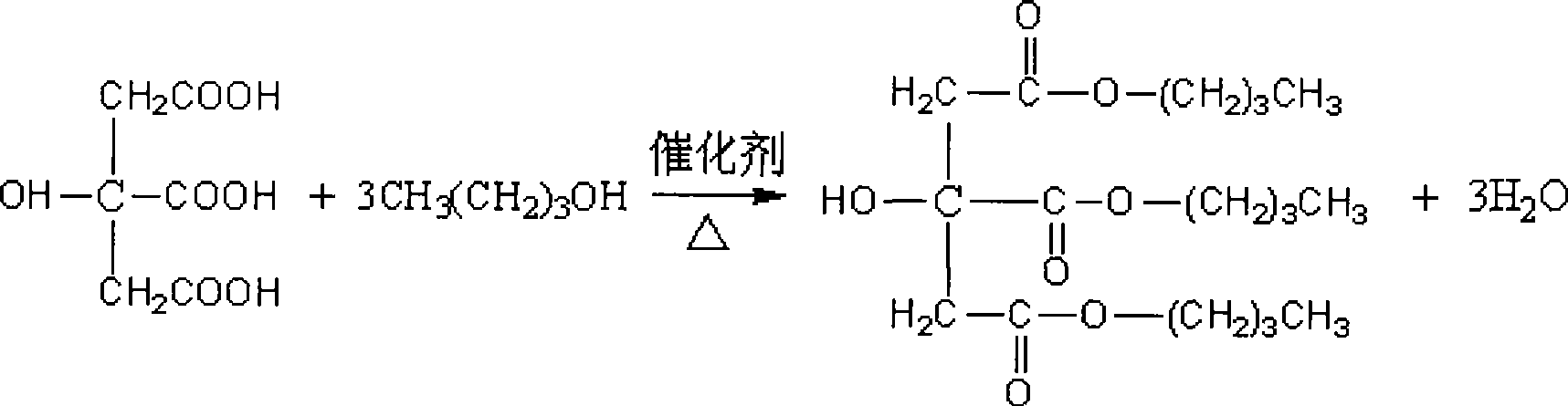
[0025] Add 0.2mol (42.03g) citric acid monohydrate and 0.8mol n-butanol to a 250mL three-necked flask equipped with electromagnetic stirring, thermometer, reflux condenser, and water separator. At the same time, add 0.63g of compound catalyst and heat to reflux and stir. , The water produced by the reaction is separated from the water trap, and the acid value is measured every one hour. The acid value is determined in accordance with GB / 1668-1995. After 6 hours of reaction, the acid value is reduced to 3.75mgKOH / g, and the esterification rate is 98.7. %, the reaction is completed, and the end temperature does not exceed 150°C.
[0026] Step 3 separation of catalyst and purification of product
[0027] Filter the reaction m...
Example Embodiment
[0028] Example 2-5
[0029] Except for the following differences, the rest is the same as in Example 1. The amount of the composite catalyst is 0.63 g, the amount of citric acid is 0.2 mol, and the amount of n-butanol is according to the proportion in Table 1, and the reaction is performed for 6 hours.
[0030] Table 1
[0031] Example Acid to alcohol ratio Esterification rate / % 2 1:3.5 97.5 3 1:4.0 98.7 4 1:4.5 98.1 5 1:5.0 97.9
Example Embodiment
[0032] Example 6
[0033] Step 1 Preparation of compound catalyst
[0034] Weigh 0.63 g of lanthanum chloride heptahydrate and p-toluenesulfonic acid in a ratio of 4:1, and mix them to obtain a binary compound catalyst.
[0035] Step 2 esterification reaction
[0036] Add 0.2mol of citric acid monohydrate and 0.9mol of n-butanol to a 250mL three-necked flask equipped with electromagnetic stirring, thermometer, reflux condenser, and water separator. At the same time, add 0.63g of compound catalyst, and heat to reflux and stir to make the reaction. The water is separated from the water separator, and the acid value is measured every hour. The acid value is measured according to GB / 1668-1995. After 7 hours of reaction, the acid value is reduced to 3.42mgKOH / g, the esterification rate is 99.1%, and the reaction is completed. , The terminal temperature does not exceed 150 ℃.
[0037] Step 3 separation of catalyst and purification of product
[0038] Filter the reaction mixture in the t...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap