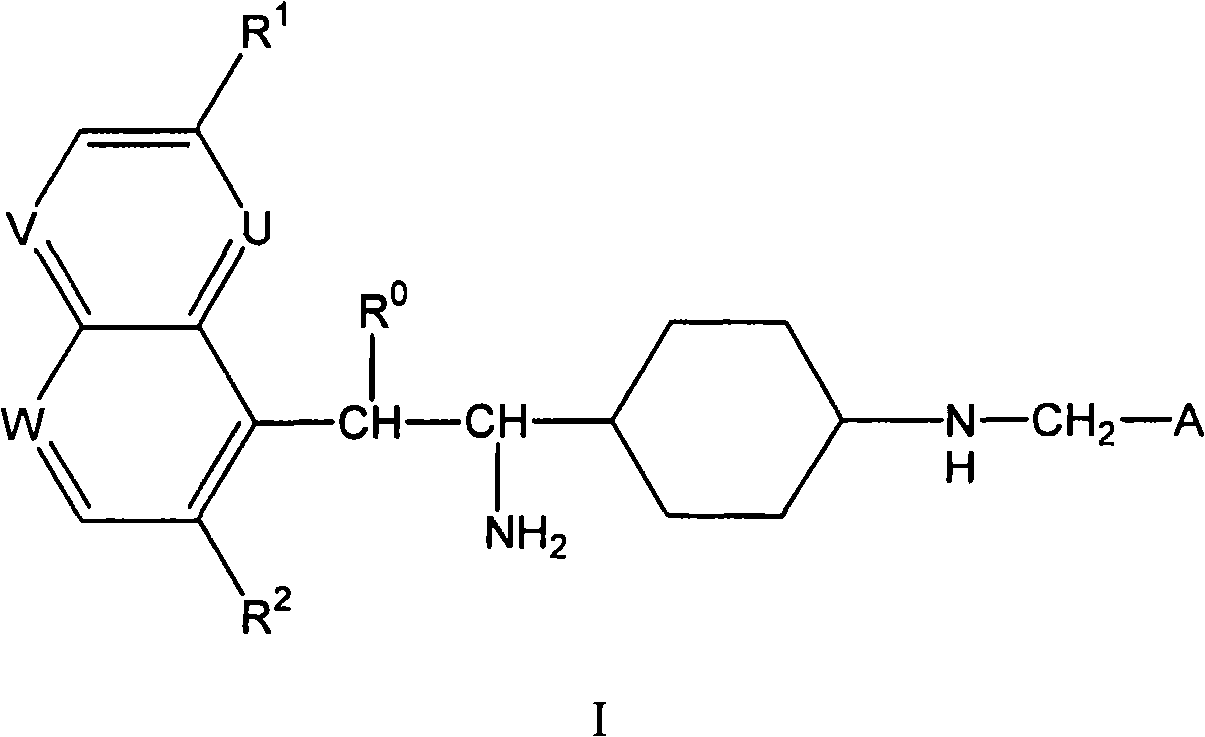
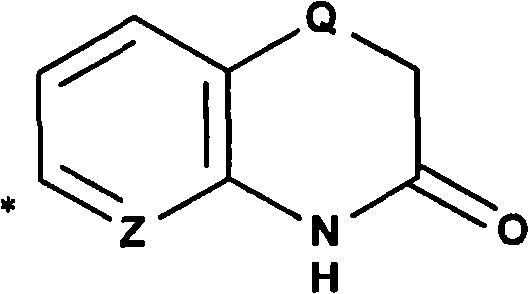
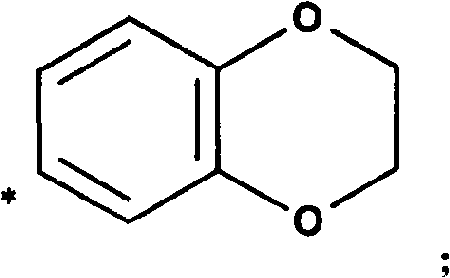
4-(1-amino-ethyl)-cyclohexylamine derivatives
A technology of cyclohexyl and cyclohexylamino, applied in the field of 4-(1-amino-ethyl)-cyclohexylamine derivatives, can solve difficult problems such as treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0645] Example 1: 6-(trans-{4-[(1R)-1-amino-2-(6-methoxy-[1,5]naphthyridin-4-yl)-ethyl]-cyclohexylamino }-methyl)-4H-pyrido[3,2-b][1,4]thiazin-3-one:
[0646] 1.i. Toluene-4-sulfonic acid trans-4-tert-butoxycarbonylamino-cyclohexylmethyl ester:
[0647] TEA (8.5 mL, 2 eq.) and p-TsCl (7 g, 1.2 eq.) were added to trans-(4-hydroxymethyl-cyclohexyl)-carbamate tert-butyl ester (7.06 g, 30.8 mmol) in DCM ( 120 mL) and THF (30 mL) in ice-cooled solution. The mixture was then stirred overnight at room temperature. DMAP (1 g) was added and the reaction was allowed to proceed for 2 hours. Add saturated NaHCO 3 (100ml). The organic layer was washed with saturated CuSO 4 (2 x 100 mL), water (100 mL) and brine for further washing. The organic layer was then concentrated to dryness. The resulting solid was filtered off, washed with water and dried under vacuum. The title tosylate was obtained as a white solid (11.7 g, 99% yield).
[0648] MS (ESI, m / z): 384.3 [M+H] + .
[0649] ...
Embodiment 2
[0697] Example 2: 6-(trans-{4-[(1S)-1-amino-2-(6-methoxy-[1,5]naphthyridin-4-yl)-ethyl]-cyclohexylamino }-methyl)-4H-pyrido[3,2-b][1,4]thiazin-3-one:
[0698] The compound of Example 2 was obtained using 2 different preparation methods.
[0699] Method A:
[0700] Starting from intermediate 1.xiv.b (0.023 g), the title enantiomer (0.018 g) was obtained as an off-white solid using the procedure described in Example 1, step 1.xv. The compound was triturated in ether.
[0701] MS (ESI, m / z): 479.2 [M+H + ].
[0702] Method B:
[0703] 2.B.i. Methanesulfonic acid (1R)-trans-1-(4-benzyloxycarbonylamino-cyclohexyl)-2-(6-methoxy-[1,5]naphthyridin-4-yl)-ethyl ester:
[0704] TEA (0.83 mL, 2 eq.), DMAP (0.036 g, 0.1 eq.) and MsCl (0.3 mL, 1.3 eq.) were added to intermediate 1.ix (1.3 g, 2.98 mmol) in DCM (30 mL) in the ice-cooled mixture. The reaction was stirred at 0 °C for 15 minutes and then at room temperature for 1 hour. Add saturated NaHCO 3 (100 mL). The two layers we...
Embodiment 3
[0718] Embodiment 3: 6-(anti-{4-[(1R * , 2R *)-1-amino-2-hydroxyl-2-(6-methoxy-[1,5]naphthyridin-4-yl)-ethyl]-cyclohexylamino}-methyl)-4H-pyrido[ 3,2-b][1,4]thiazin-3-one:
[0719] 3.i. trans-2-(4-tert-butoxycarbonylamino-cyclohexyl)-3-hydroxy-3-(6-methoxy-[1,5]naphthyridin-4-yl)-propionic acid Methyl esters:
[0720] LiHMDS (1M in THF, 17.1 mL) was added dropwise to trans-(4-tert-butoxycarbonylaminocyclohexyl)-acetic acid methyl ester (1.80 g, 6.63 mmol; prepared according to WO 2000 / 024717) over 10 minutes ) in THF (20 mL) cooled to -78°C. The resulting solution was stirred in a dry ice bath (set the temperature at -40°C) for 1.5 hours. The reaction was recooled to -78°C and 6-methoxy-[1,5]naphthyridine-4-carbaldehyde (3.35 g, 17.82 mmol; prepared according to WO 2006 / 032466) was added rapidly as a solid (2 mL was also added THF for washing) and stirring was continued at -78°C for 1.75 hours. Add NH 4 Cl (50 mL) and EA (50 mL). The two layers were separated and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com