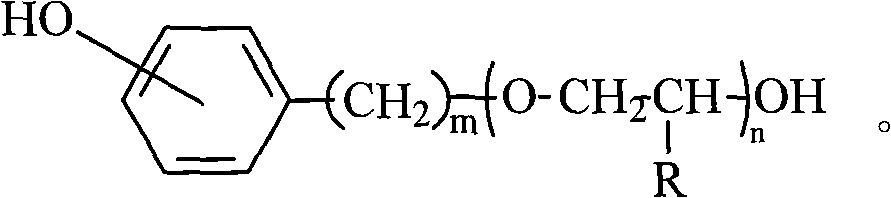Asymmetric polyether dihydric alcohol and preparation method thereof
A polyether diol, asymmetric technology, applied in the field of asymmetric polyether diol, to achieve the effect of reducing the degree of dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
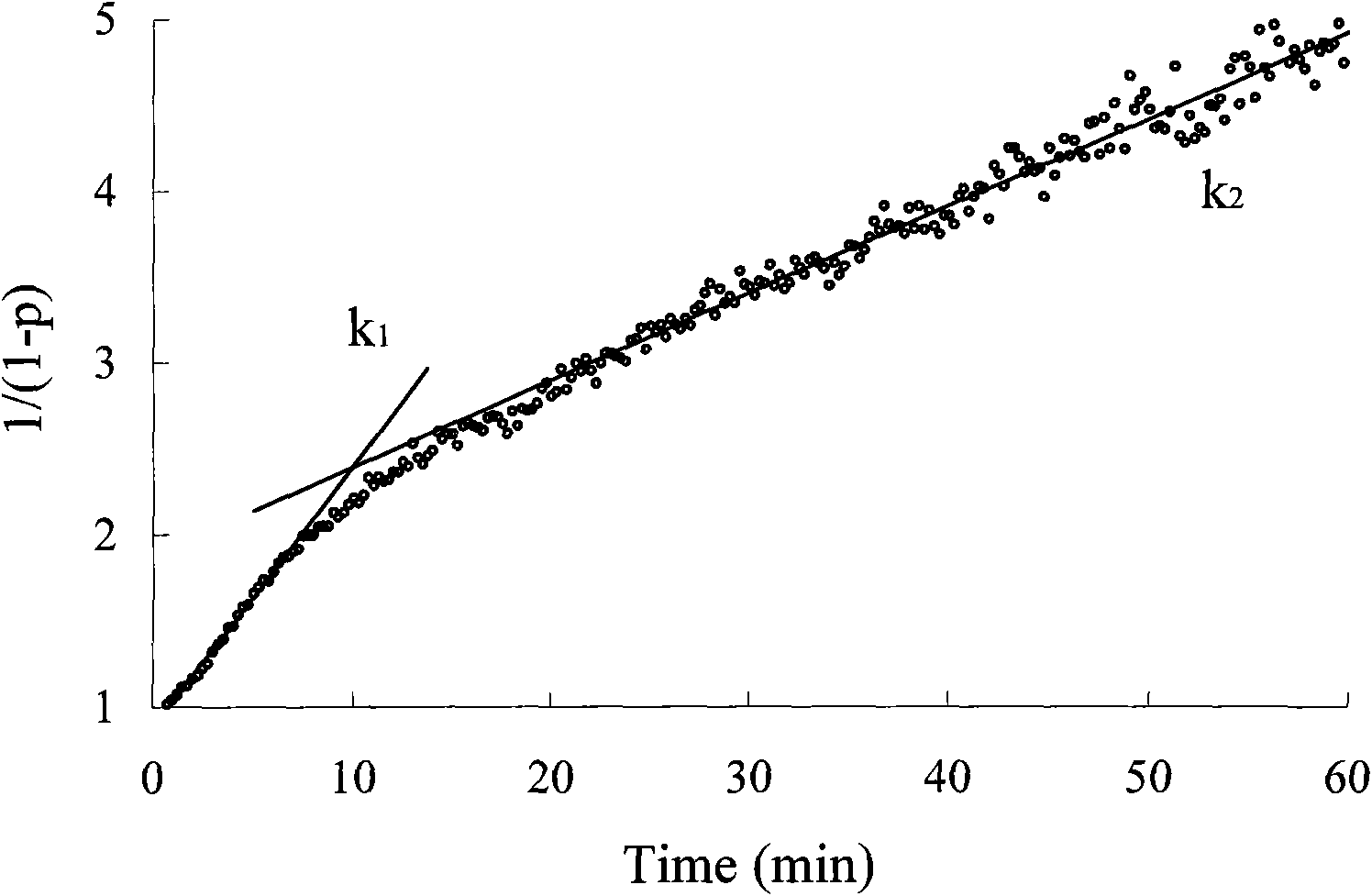
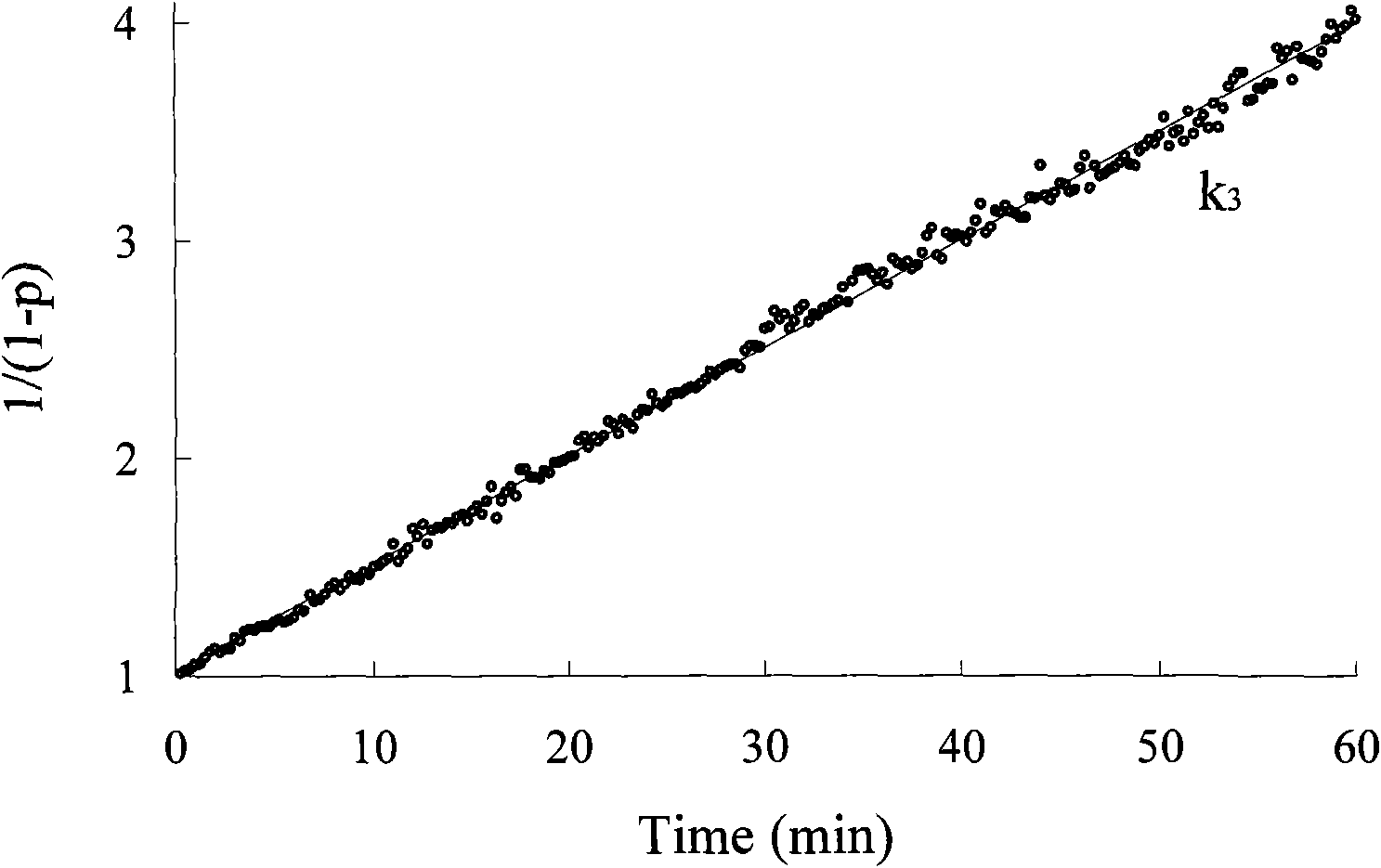
Image
Examples
Embodiment 1
[0024] [Example 1] unsymmetrical polypropylene oxide diol
[0025] A, the synthesis of p-benzyloxyphenethyl alcohol
[0026] 16.6 grams of p-hydroxyphenethyl alcohol and 20.7 grams of potassium carbonate were dispersed in 500 milliliters of acetone, and 17.1 grams of benzyl bromide was added. The mixture was then refluxed at the boiling point of acetone (56° C.) for 6 hours, and the reaction was completed. The mixture was first concentrated to remove the solvent, filtered to remove potassium carbonate, washed three times with 3 mol / L potassium hydroxide solution, and finally washed with distilled water until neutral. The obtained solid was dried to constant weight under vacuum to obtain 18.2 g of p-benzyloxyphenethyl alcohol solid powder.
[0027] After analysis, the infrared and NMR data of p-benzyloxyphenethyl alcohol are: IR(cm -1 ): 3294.42, 3030.17, 2858.51, 1608.63, 1512.19, 1452.40, 1384.89, 1236.37, 1174.65, 1055.06, 1010.70. 1 H NMR δ(DMSO-d6): 2.64(t, 2H, C H 2...
Embodiment 2
[0035] [Example 2] unsymmetrical polypropylene oxide diol
[0036] A, the synthesis of p-benzyloxyphenethylate potassium
[0037] Disperse 10.0 grams of p-benzyloxyphenylpropanol and 2.96 grams of potassium tert-butoxide in 300 milliliters of cyclohexane, then heat the mixture to the azeotropic point of cyclohexane and tert-butanol, and use cyclohexane by azeotropic distillation Alkanes take tert-butanol out. When the distillate reaches 100 ml, stop the reaction. The remaining solvent was distilled off under dry conditions to obtain 10.9 grams of solid powder of potassium p-benzyloxyphenethylate.
[0038] C. Propylene oxide polymerization reaction
[0039] Add 10.1 grams of potassium p-benzyloxyphenethylate and 39.5 grams of propylene oxide obtained in the previous step into the closed reactor, heat to 35° C. and stir, and the polymerization ends after 8 hours. Distilled water was added to terminate the reaction and the pH value was adjusted to neutral with dilute hydrochl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com