Benzamide histone deacetylase inhibitor and application thereof
A technology of phenyl and compound, which is applied in the field of benzamide histone deacetylase inhibitors and its application, and can solve the problems of high toxicity, few types of drugs, and inaccurate curative effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] The biological activity research of embodiment 1 compound of the present invention
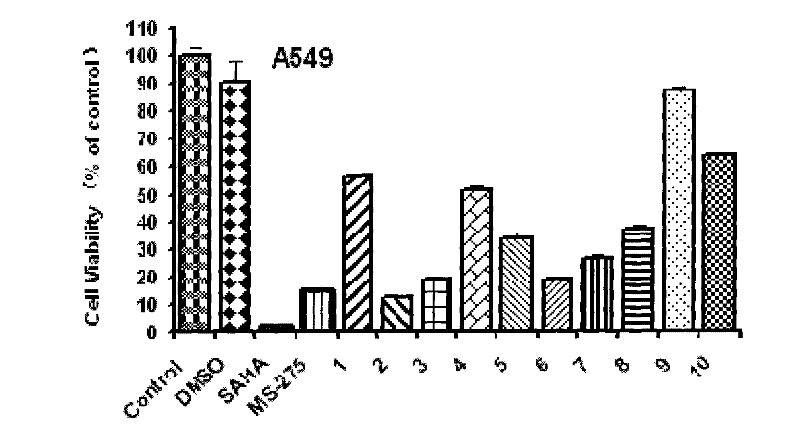
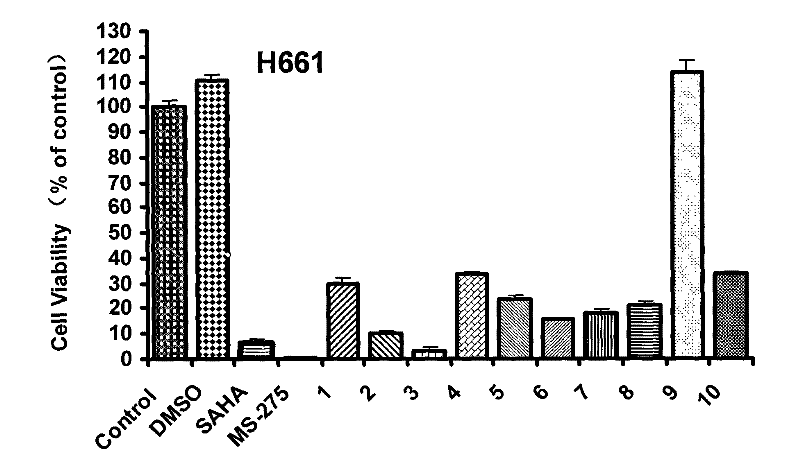
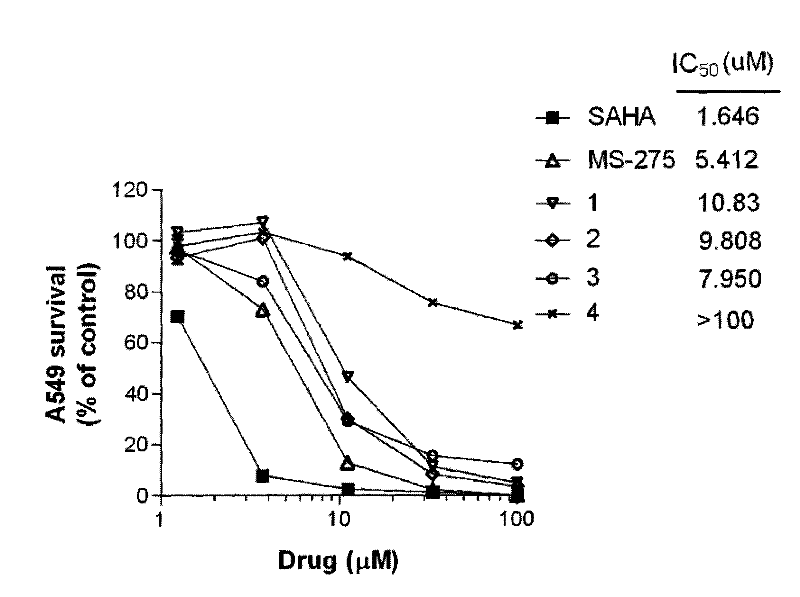
[0075] Investigate the compound of the present invention that obtains in the embodiment 2~15 of different concentrations to human non-small cell lung adenocarcinoma cell line (A549) and human large cell lung cancer cell (NCI-H661) after acting 72h, WST-8 method detects that the drug has the effect on the cell. effects on proliferation.
[0076] Drugs: The positive drugs SAHA and MS-275 were synthesized by our laboratory.
[0077] Reagents and instruments: HyQR modified RPMI 1640 medium, DMEM medium, WST-8 (Sigma), electron coupling reagent 1-Methoxy PMS (Sigma), trypsin. CO 2 Incubator, sterile operating table, microplate reader, centrifuge, pipette gun, pipette, centrifuge tube and 96-well plate, etc.
[0078] method:
[0079] 1) Tumor cell line culture
[0080] Human large cell lung cancer cells (NCI-H661): use RPMI 1640 medium containing 10% fetal bovine serum at 37°C, 5% CO 2 ...
Embodiment 2
[0096] Example 2 Preparation of 3-phenyl-5-(chloromethyl)-1,2,4-oxadiazole
[0097]
[0098] Aniline oxime (13.6 g, 0.10 mol) was dissolved in acetone (300 mL), and anhydrous potassium carbonate (13.8 g, 0.10 mol) was added. A solution of chloroacetyl chloride (8.07 mL, 11.3 g, 0.10 mol) in acetone (10 mL) was added dropwise over 75 min at 0°C. The reaction mixture was warmed to room temperature and stirred for 1 h. The solvent was distilled off under reduced pressure to obtain a white solid. The solid was dissolved in ethyl acetate (400 mL), and the organic layer was successively washed with water (3×100 mL), washed with saturated brine (3×100 mL), and dried over anhydrous magnesium sulfate. After filtration, the solvent was distilled off under reduced pressure to obtain 23.9 g of a white powdery solid O-acylated product (93%).
[0099] The O-acylated product (22.9 g, 0.089 mol) was suspended in 300 mL of xylene and refluxed for 3 h. The solvent was distilled off under...
Embodiment 3
[0101] Example 3 Preparation of methyl 4-{[(3-phenyl-1,2,4-oxadiazol-5-yl)methyl]aminomethyl}benzoate
[0102]
[0103] Dissolve 0.015mol of methyl p-aminomethylbenzoate in 50mL of DMF, add 0.025mol of anhydrous potassium carbonate, dropwise add 0.01mol of 3-phenyl-5-(chloromethyl)-1,2,4 - Oxadiazole, stirred at 40°C for 8h. Then it was poured into ice water, extracted with ethyl acetate (3×50 mL), dried over anhydrous magnesium sulfate, and the product could be obtained by column chromatography with a yield of 55%.
[0104] Mp: 41-43°C. 1 H-NMR (500Hz, CDCl3): δ8.08-8.10(m, 2H), 8.01((d, J=8.2Hz, 2H), 7.47-7.51(m, 3H), 7.44((d, J=8.2 Hz, 2H), 4.11 (s, 2H), 3.98 (s, 2H), 3.90 (s, 3H), 2.10 (br s, 1H). 13 C-NMR (125Hz, CDCl3): δ178.03, 168.31, 166.84, 144.18, 131.22, 129.83, 129.30, 128.86, 128.83, 128.10, 127.47, 127.44, 126.62, 52.58, 51.99, 43.96. MS: (M+H)+324.1, (M+Na)+346.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com