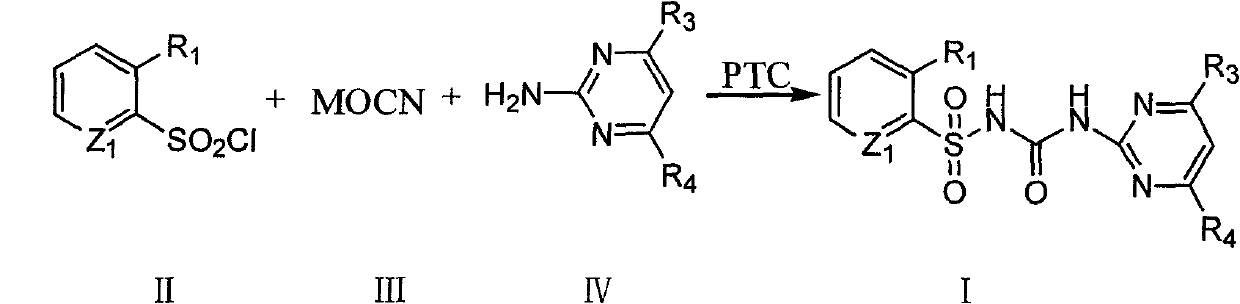
Method for synthesizing sulfonylureas compound under phase-transfer catalysis
A technology of phase-transfer catalysis and phase-transfer catalyst, which is applied in the field of synthesis of sulfonylurea compounds, can solve the problems of many steps, high production costs, and expensive raw materials, and achieve the effects of increasing yield, easy operation, and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Preparation of 2-(4,6-dichloropyrimidin-2-yl)-1-(2-methoxycarbonylbenzene-1-yl)sulfonylurea
[0023] Weigh 23.4g of 2-methoxycarbonyl-1-benzenesulfonyl chloride into the reactor, add 100mL of acetonitrile, then weigh 6.6g of NaOCN, add 0.8g of n-butylammonium bromide into the reaction solution, stir at room temperature for 1.5 After 1 h, add 16.4 g of 4,6-dichloro-2-aminopyrimidine, heat to reflux for 5 h, after the reaction is completed, cool to room temperature, evaporate acetonitrile under reduced pressure, add water and stir for 0.5 h, filter to obtain a white solid, wash with methanol, and dry After drying, about 29.9 g of 2-(4,6-dichloropyrimidin-2-yl)-1-(2-methoxycarbonylphen-1-yl)sulfonylurea was obtained, with a purity of 92% and a yield of 80.9%.
Embodiment 2
[0024] Example 2: 2-(4,6-dimethoxypyrimidin-2-yl)-1-(3-dimethylcarbamoylpyridin-2-yl)sulfonylurea
[0025] Weigh 24.8g of 3-dimethylcarbamoyl-2-sulfonyl chloride pyridine and place it in the reactor, add 100mL of toluene, 6.6g of NaOCN, and 0.92g of benzyltriethylammonium chloride into the reaction solution. After stirring at room temperature for 1 h, 155.16 g of 4,6-dimethoxy-2-aminopyrimidine was added, and the following operations were the same as in Example 1 to obtain the product 2-(4,6-dimethoxypyrimidin-2-yl)-1- (3-Dimethylcarbamoylpyridin-2-yl)sulfonylurea 39.6g, purity 94.4%, yield 91.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com