Novel production process of polyferric chloride
A technique for polymerizing ferric chloride and ferrous chloride, applied in ferric halides, chemical instruments and methods, separation methods, etc., can solve the problems of violent reaction, high production cost, high cost of chlorate direct oxidation method, etc., and achieve high quality High, long storage time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0028] Example 1
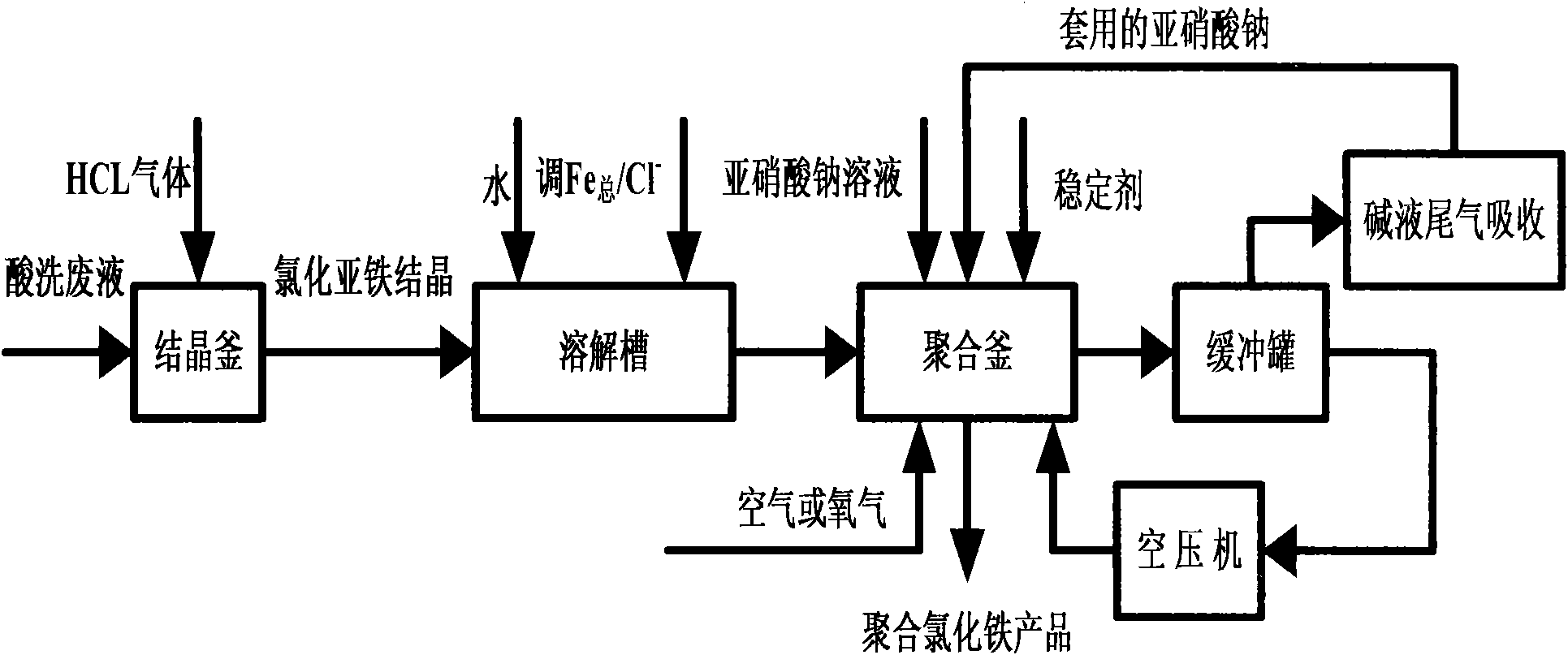
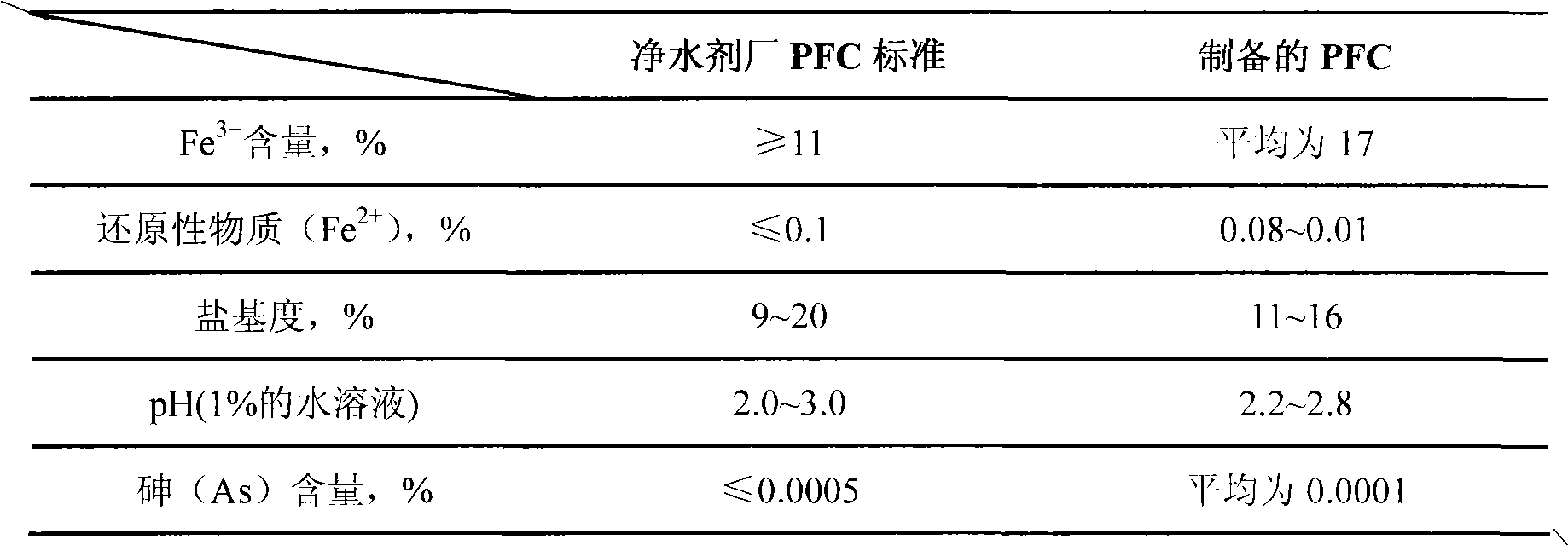
[0029] Take 40ml of the pickling waste liquid from the pickling section of the steel plant (the pickling waste liquid is taken from a steel plant, and the appearance is a yellow-green clear liquid with a sour taste, [H + ]=9.8mol / L, total iron content is 154.9g / L, of which ferric ion content is 149.1g / L, HCl acidity is 12.07%) added to a three-necked flask for reaction. Inject continuously stable HCl gas into the pickling waste liquid. The other mouth of the three-necked bottle is connected to a buffer bottle to prevent back suction. After the buffer bottle, use the pickling waste liquid for circular absorption.
[0030] In order to prevent the reaction from being too rapid, HCl gas was introduced slowly and stably while stirring the waste pickling liquid for full absorption. Depending on the aeration rate, about 20 to 60 minutes, yellow-green crystals were continuously precipitated in the waste pickling liquid. HCl gas is continuously introduced and cooled to 1...
Example Embodiment
[0032] Example 2
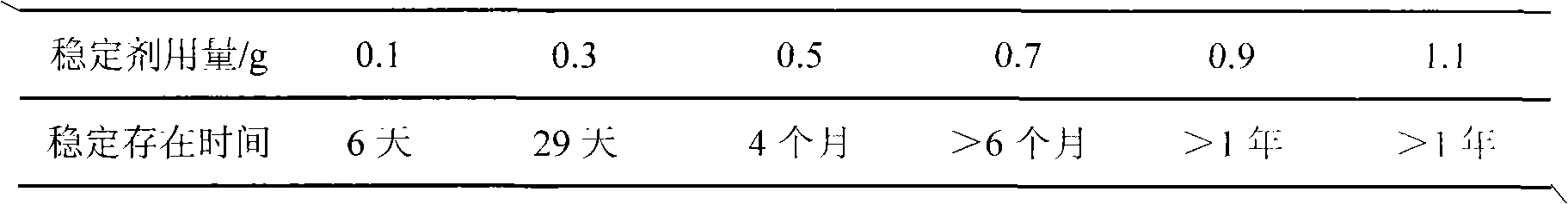
[0033] Take 40ml of the pickling waste liquid from the pickling section of the steel plant (the pickling waste liquid is taken from a steel plant, the appearance is a yellow-green clear liquid with a sour taste, and the total iron content is 154.9g / L) and add it to a three-necked bottle. In the same way as in Example 1, HCl gas was introduced for pretreatment. After treatment, 16.33g of ferrous chloride crystals (not dried) are added to 100ml of water to stir and dissolve, firstly add 0.64g of iron filings to adjust Fe total / Cl - Then add 0.86g sodium dihydrogen phosphate stabilizer to adjust P / Fe total Continue to stir and mix, and then add 0.7g of sodium nitrite solid at one time, and pass in air or oxygen; start heating, keep it at 50°C for reaction. At the same time, open the forced gas circulation system centered on the gas buffer tank, and the system is full of red-brown NO 2 Gas, polymerization reaction occurs, the reaction lasts for about 1.5 to 2 hour...
Example Embodiment
[0034] Example 3
[0035] Take 40ml of the pickling waste liquid from the pickling section of the steel plant (the pickling waste liquid is taken from a steel plant, the appearance is a yellow-green clear liquid with a sour taste, and the total iron content is 154.9g / L) and add it to a three-necked bottle. In the same way as in Example 1, HCl gas was introduced for pretreatment. After treatment, 16.33g of ferrous chloride crystals (not dried) are added to 100ml of water to stir and dissolve, firstly add 0.64g of iron filings to adjust Fe total / Cl - Then add 0.86g sodium dihydrogen phosphate stabilizer to adjust P / Fe total Continue to stir and mix well, then add 0.7g of sodium nitrite solid in two batches, 0.35g for each batch, with an interval of 0.5 hours, pass in air or oxygen; start heating, and keep at 50℃ for reaction. At the same time, open the forced gas circulation system in the system centered on the gas buffer tank, and the system is full of red-brown NO 2 Gas, polymer...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap