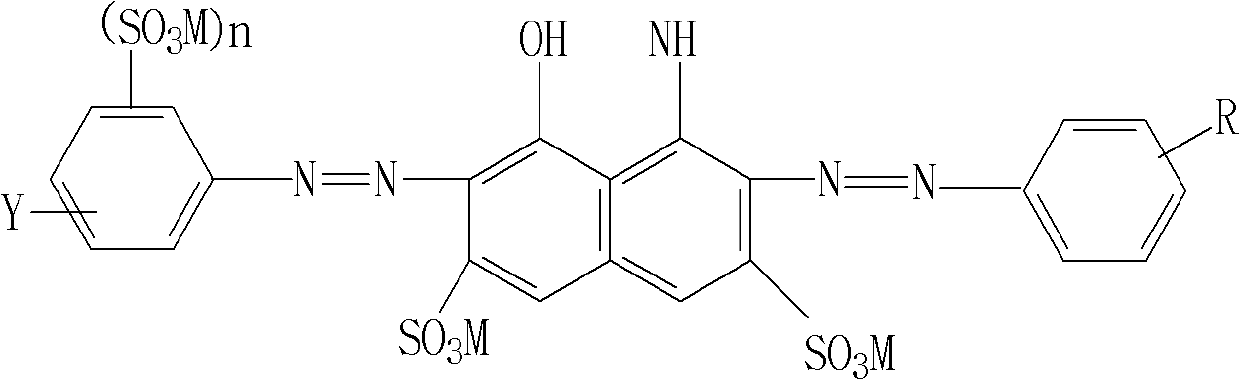
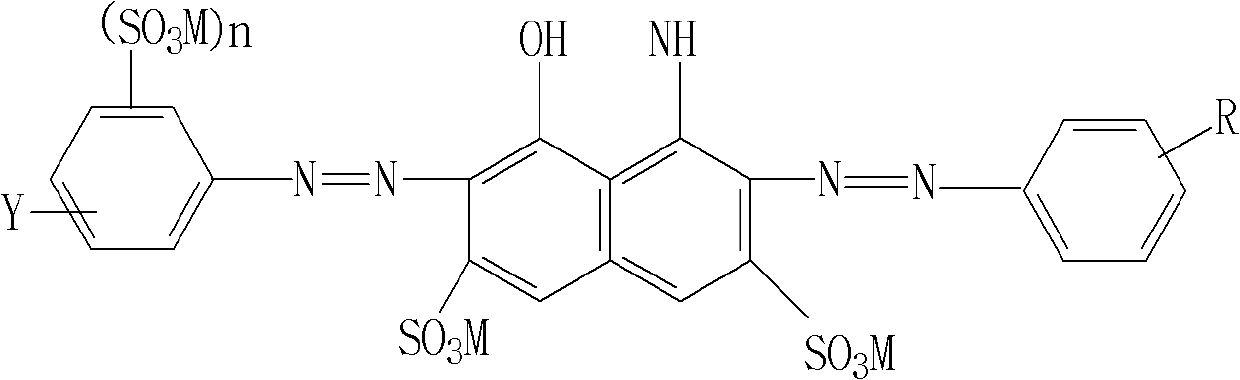
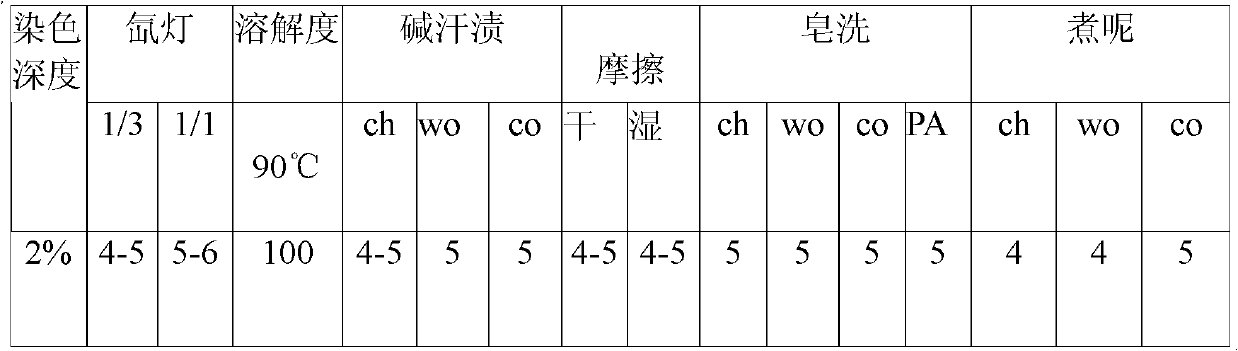
Reactive navy blue dye and preparation method thereof
A technology of reactive dyes and reactions, applied in reactive dyes, dyeing methods, azo dyes, etc., can solve problems such as poor fastness, and achieve the effects of improved fastness, extremely high reject rate, and low raw material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] a. meta-ester diazotization reaction
[0043] Add bottom water to the reaction kettle, add 15 parts of meta-ester, stir to dissolve, add quantitative 30% hydrochloric acid solution, add crushed ice to make the solution C=10-15%, then add 30% nitrous acid sodium solution. Make the pH in the reaction solution > 2.0, and the starch potassium iodide test paper will be slightly blue after dipping. T=0-5°C, react for 2 hours. Use sulfamic acid to eliminate excess nitrous acid, and wait for acidic coupling.
[0044] b. Acid coupling reaction
[0045] Take 33.5 parts of H acid, neutralize pH=6.0-7.0 with 20%-40% sodium hydroxide solution, dissolve it completely, add it to the meta-ester diazonium salt, control T=0-5°C, and react 6 -10 hours, after passing the TCL chromatographic analysis, wait for alkaline coupling.
[0046] c. Preparation of Condensate
[0047] Add a small amount of bottom water into the reaction kettle, add crushed ice cubes, then add 35 parts of sodium...
Embodiment 2
[0058] a. The diazotization reaction of sodium p-aminobenzenesulfonate
[0059] Add bottom water to the reaction kettle, add 13.8 parts of sodium p-aminobenzenesulfonate, stir to dissolve, add quantitative 30% hydrochloric acid solution, add crushed ice cubes, make C=5-10% in the solution, and then add 30% of sodium nitrite solution. The pH in the reaction solution is greater than 2.0, and the starch potassium iodide test paper is slightly blue after soaking. T=0-5°C, react for 1-3 hours. Use sulfamic acid to eliminate excess nitrous acid, and wait for acidic coupling.
[0060] b. Acid coupling reaction
[0061] Add 32.7 parts of H acid to p-nitroaniline diazonium salt, control T=10-20°C, react for 6-10 hours, and wait for alkaline coupling after passing the TCL chromatography analysis.
[0062] c. Para-ester diazotization reaction
[0063] Add bottom water to the reaction kettle, add 27.5 parts of para-ester, stir to dissolve, add quantitative 30% hydrochloric acid solut...
Embodiment 3
[0074] a Add a small amount of bottom water to the reaction kettle, add crushed ice cubes, then add 35 parts of sodium 2,4-diaminobenzenesulfonate, add 24 parts of cyanuric chloride dropwise while stirring, T=0-5°C, use 10%-20% sodium carbonate solution is used to control the pH=6.0-7.5. After reacting for 4-6 hours, after the TLC plate confirms that it is qualified, the condensate is diazotized. .
[0075] b. Adjust the temperature of the condensate solution T=5-12, add quantitative 30% hydrochloric acid solution, and then add 30% sodium nitrite solution. Make the pH in the reaction solution > 2.0, and the starch potassium iodide test paper will be slightly blue after dipping. T=15-20°C, react for 1-3 hours. Use sulfamic acid to eliminate excess nitrous acid, and wait for acidic coupling.
[0076] b. Acid coupling reaction
[0077] Take 33.5 parts of H acid, use 20%-40% sodium hydroxide solution to neutralize pH=6.0-7.0, and make it completely dissolved. Add it dropwise t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com