Preparation method of tamibarotene stable crystals
A tamibarotene and crystallization technology, which is applied in the field of compound crystal preparation, can solve the problems of strong irritating odor, easy residual solvent, strong corrosiveness, etc., and achieves a solution with no solvent residue, low reagent toxicity, and avoidance of degradation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
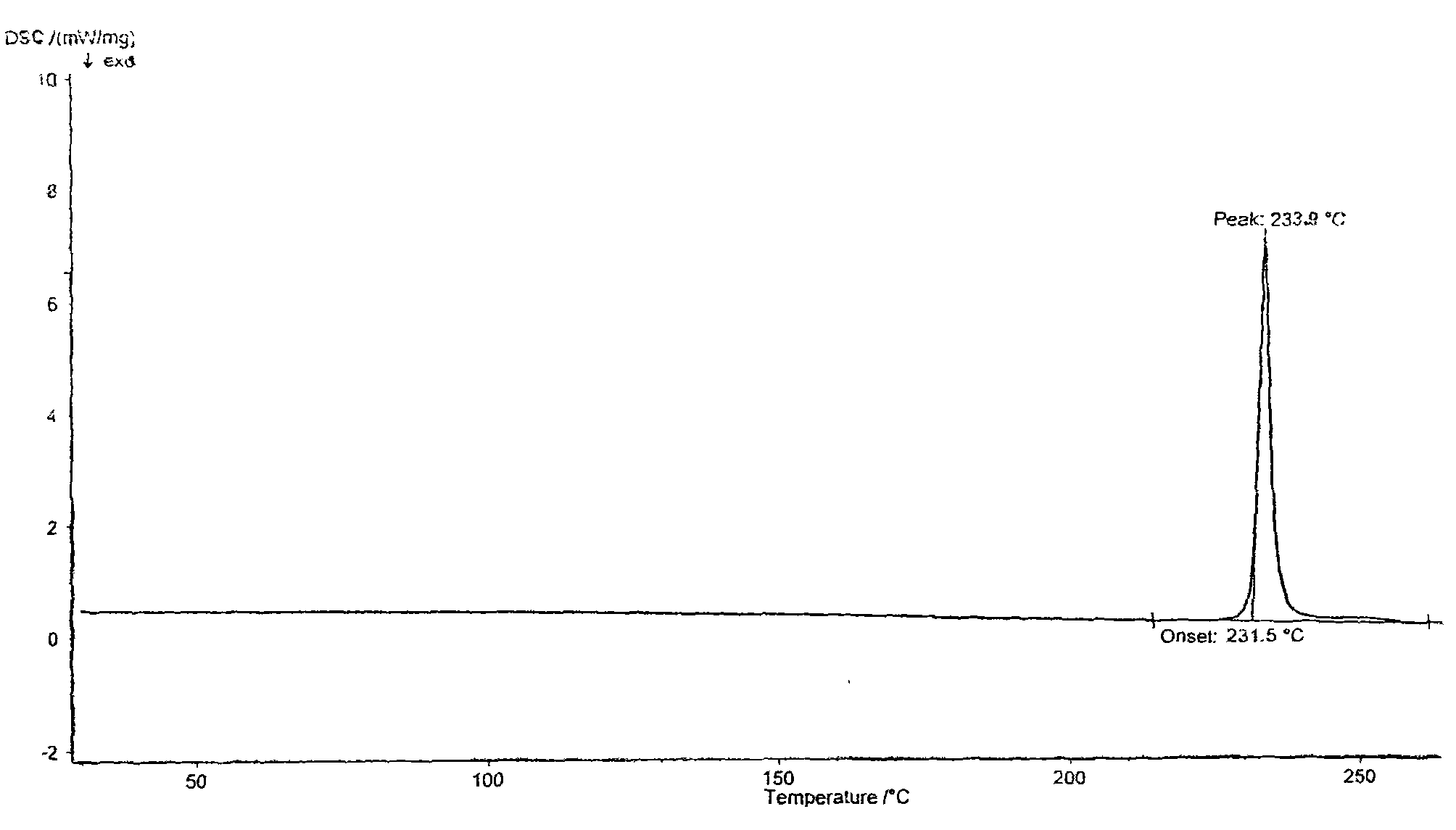
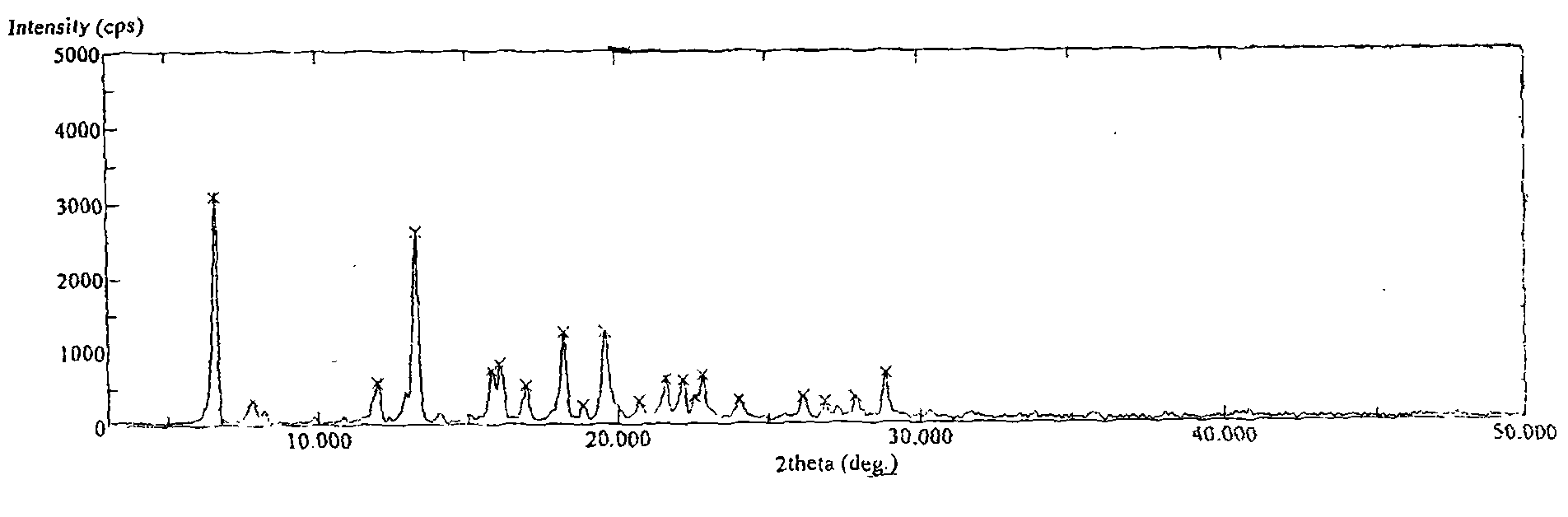
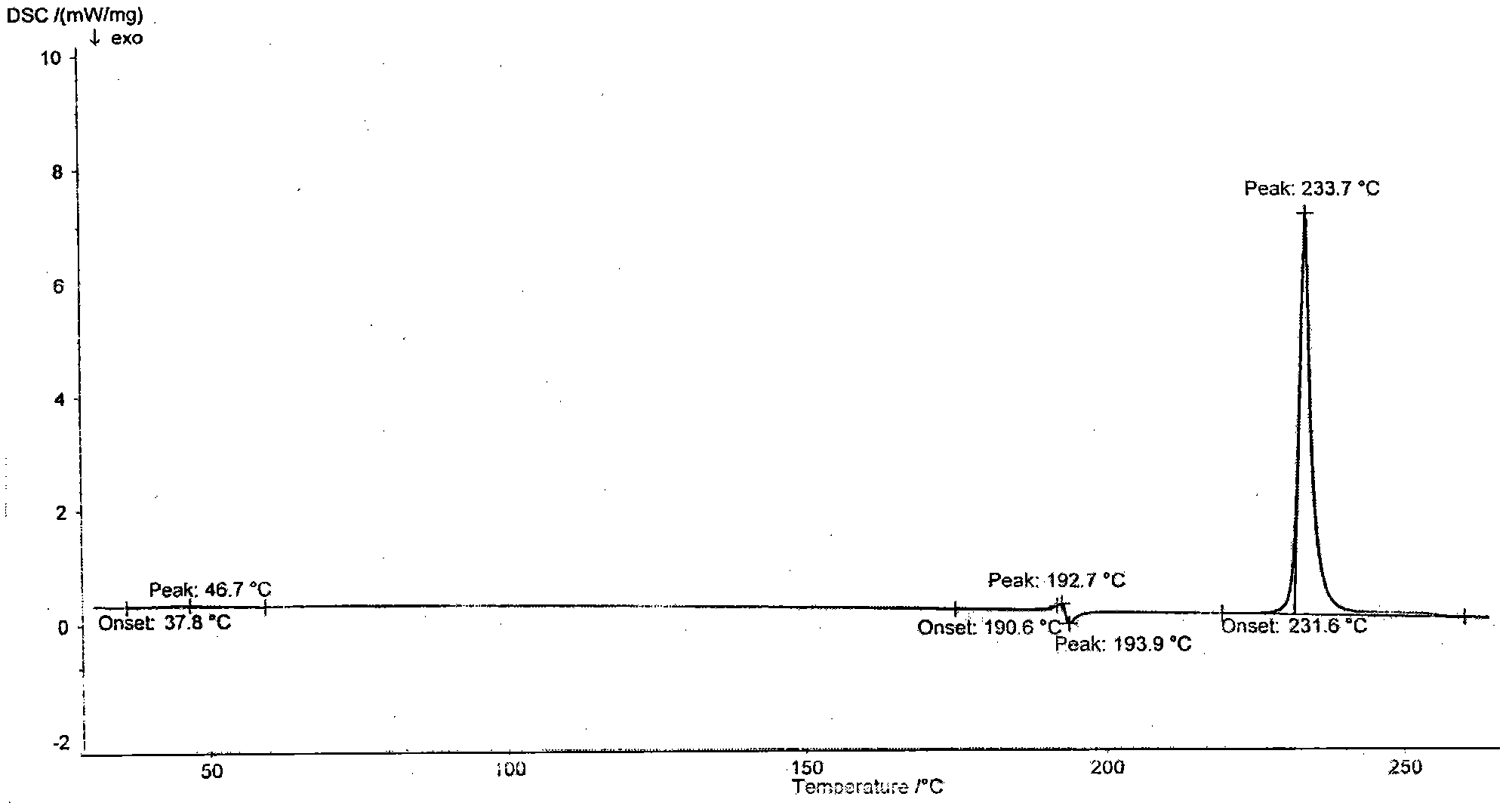
[0020] Add 2 g of tamibarotene into 60 ml of methanol-20 ml of water mixed solvent, reflux for 0.5 hours, let it stand and cool to room temperature, fully analyze the crystallization, filter and dry the crystals in vacuum at 80°C for 4 hours, and crush the dried needle crystals into In fine powder form, vacuum-dried at 140° C. for 24 hours to obtain 1.85 g of Tamibarotene crystals. The melting point measured by capillary method is 233-234°C. The purity detected by HPLC was 99.83%. Simple impurities are all below 0.1%.
Embodiment 2
[0022] Add 2 g of tamibarotene into 40 ml of methanol-20 ml of water mixed solvent, reflux for 0.5 hours, let it stand to cool to room temperature, fully analyze the crystallization, filter and dry the crystals in vacuum at 80°C for 4 hours, and crush the dried needle crystals into In fine powder form, vacuum-dried at 160° C. for 15 hours to obtain 1.79 g of Tamibarotene crystals. The melting point measured by capillary method is 233-234°C. The purity by HPLC was 99.85%. Simple impurities are all below 0.1%.
Embodiment 3
[0024] Add 2 g of tamibarotene into 40 ml of methanol-10 ml of water mixed solvent, reflux for 0.5 hours, let it stand and cool to room temperature, fully analyze the crystallization, filter and dry the crystals in vacuum at 80°C for 4 hours, and crush the dried needle crystals into In fine powder form, vacuum-dried at 170° C. for 10 hours to obtain 1.80 g of Tamibarotene crystals. The melting point measured by capillary method is 233-234°C. The purity detected by HPLC was 99.80%. Simple impurities are all below 0.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com