Non-aqueous electrolyte, preparation method thereof and battery adopting electrolyte
A non-aqueous electrolyte and electrolyte technology, applied in secondary batteries, circuits, electrical components, etc., can solve the problem that the electrolyte cannot obtain reversible overcharge protection and flame retardancy at the same time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The present invention also provides a kind of preparation method of non-aqueous electrolytic solution, this method comprises:
[0030] 1) Put C 1 -C 4 Alcohol is mixed with phosphorus oxychloride to generate monochlorophosphate at 15°C;
[0031] 2) Mix the monochlorophosphate formed in step (1) with substituted phenothiazine and tetra-n-butylammonium hydrogen sulfate, and then stir and reflux at room temperature to obtain the additive with the following structure
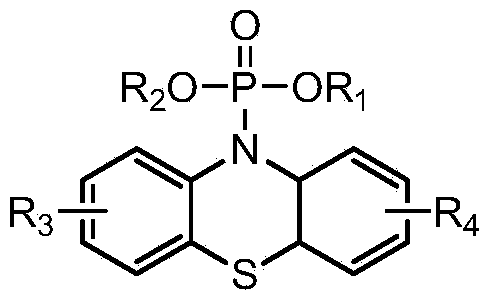
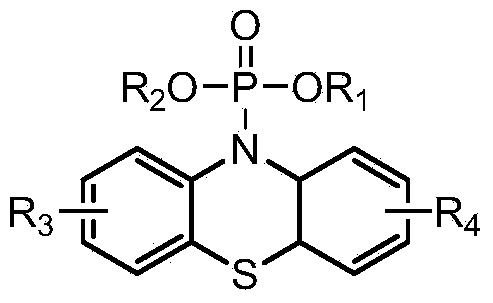
[0032]
[0033] Wherein, R1, R2 are independently selected from alkene group, aromatic hydrocarbon group and C 1 -C 12 One of the alkyl groups, or containing F and / or N atom alkenyl group, aromatic hydrocarbon group and C 1 -C 12 One of the alkyl groups; R3 and R4 are independently selected from one of hydrogen atom, halogen atom, acetyl group, trifluoromethyl group, carboxylate group, cyano group and nitro group;
[0034] 3) Mix the lithium salt and the organic solvent, and then add the additive pre...
Embodiment 1
[0045] This example is used to illustrate the additives, electrolyte, positive and negative electrodes and their preparation methods provided by the present invention.
[0046] (1) Preparation of additives and electrolytes added with such additives:
[0047] Add 7.75g of phosphorus oxychloride to a 500ml two-necked flask, add 3.2g of anhydrous methanol at a rate of 0.5g / min, stir magnetically at 80r / min, and adjust the temperature to 15°C with ice-salt water. After the dropwise addition of anhydrous methanol is completed, keep warm for 1 h.
[0048] Pour the above reaction solution into a distillation bottle, carry out vacuum distillation (55°C), collect the fractions to obtain monochlorophosphate, and mix 8.375g of monochlorophosphate with 12.07g of 2-acetylphenothiazine, 0.169g Dissolve tetra-n-butylammonium hydrogen sulfate together in 100mL of methyl isopropyl ketone, add 50mL of 50% sodium hydroxide aqueous solution under stirring, stir at room temperature for 24h and th...
Embodiment 2
[0059] This example is used to illustrate the additives, electrolyte, positive and negative electrodes and their preparation methods provided by the present invention.
[0060] Add 7.75g of phosphorus oxychloride to a 500ml two-necked flask, add 4.6g of absolute ethanol at a rate of 0.5g / min, stir magnetically at 80r / min, and adjust the temperature to 15°C with ice-salt water. After the absolute ethanol was added dropwise, keep warm for 1 h.
[0061] Pour the above reaction solution into a distillation bottle, carry out vacuum distillation (55°C), collect the distillate to obtain monochlorophosphate, and mix 10.75g of monochlorophosphate with 11.22g of 2-methylcyanophenothiazine, 0.169 g of tetra-n-butylammonium hydrogen sulfate were dissolved in 100mL of methyl isopropyl ketone, and 50mL of 50% sodium hydroxide aqueous solution was added under stirring, stirred at room temperature for 24h and then refluxed for 24h.
[0062] Pour the reaction solution into a 1000mL separatory...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com