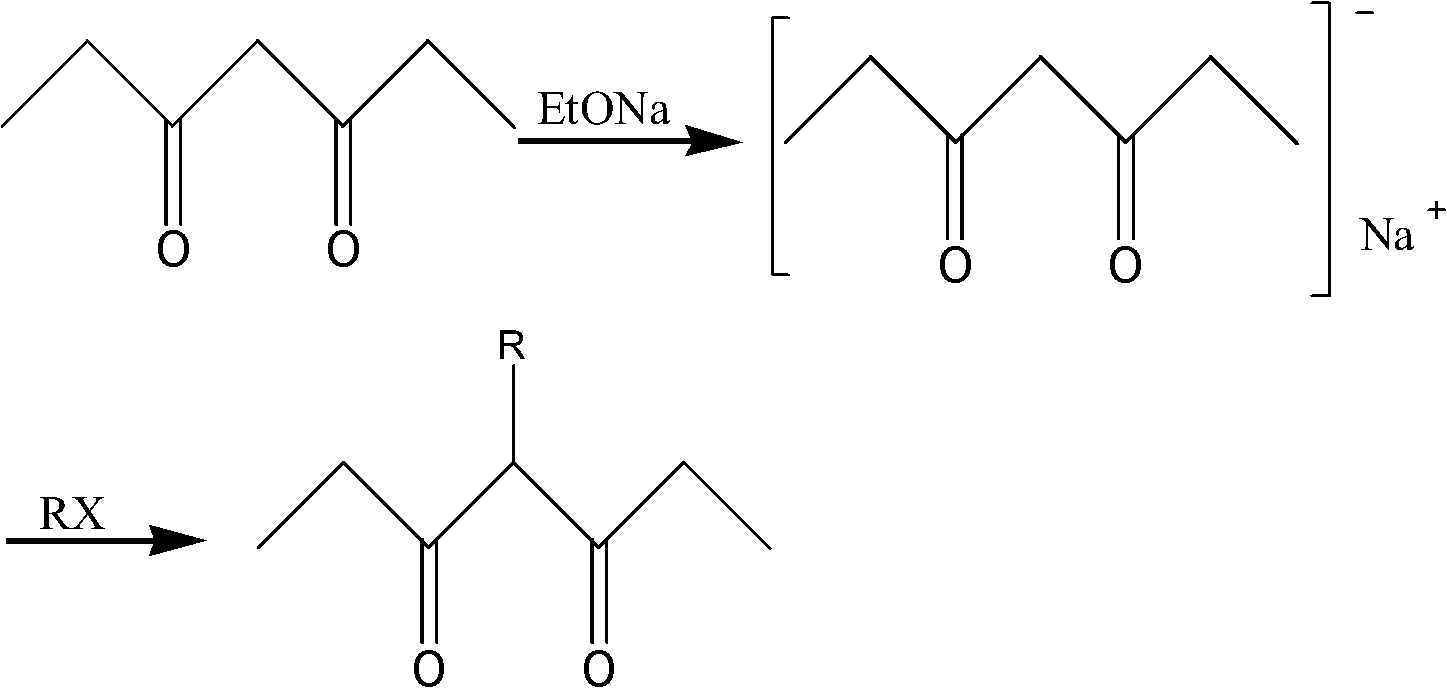
Synthetic method of 4-R-3,5-heptadione
A synthetic method, the technology of heptanedione, which is applied in the field of chemical synthesis, can solve the problems of high cost, low product yield, and difficult recycling of solvents, etc., and achieve the effects of short reaction cycle, high yield, and easy control of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Suspend 85g of sodium ethylate with a mass fraction of 90% in 200ml of benzene in a reaction flask, add 1.2mol of 3,5-heptanedione dropwise into the reaction flask, react for 1 hour and cool to room temperature, add 1.5mol of bromoethane Then, react for 3 hours. After the reaction was completed, the pH value was adjusted to 7.0 with dilute hydrochloric acid, the reaction solution was allowed to stand and separated, and the organic layer was separated. The organic layer was dried with anhydrous sodium sulfate, and the solvent was recovered to finally obtain 0.66 mol of the product 4-ethyl-3,5-heptanedione with a yield of 55%.
Embodiment 2
[0018] Suspend 85g of sodium ethoxide with a mass fraction of 90% in 200ml of toluene in a reaction flask, add 1.2mol of 3,5-heptanedione dropwise into the reaction flask, react for 1 hour and cool to room temperature, add 1.5mol of bromoethane Then, react for 3 hours. After the reaction was completed, the pH value was adjusted to 7.0 with dilute hydrochloric acid, the reaction solution was allowed to stand and separated, and the organic layer was separated. The organic layer was dried with anhydrous sodium sulfate, and the solvent was recovered to finally obtain 0.624 mol of the product 4-ethyl-3,5-heptanedione with a yield of 52%.
Embodiment 3
[0020] Suspend 85g of sodium ethoxide with a mass fraction of 90% in 200ml of xylene in a reaction flask, add 1.2mol of 3,5-heptanedione dropwise into the reaction flask, react for 1 hour and cool to room temperature, add 1.5mol of ethyl bromide After alkane, react for 3 hours. After the reaction was completed, the pH value was adjusted to 7.0 with dilute hydrochloric acid, the reaction solution was allowed to stand and separated, and the organic layer was separated. The organic layer was dried with anhydrous sodium sulfate, and the solvent was recovered to finally obtain 0.636 mol of the product 4-ethyl-3,5-heptanedione with a yield of 53%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com