Method for purification and preparation of mono-substituted PEG-EPO (polyethylene glycol-erythropoietin)
A PEG-EPO, single substitution technology, applied in the purification process and preparation field of single substitution PEG-EPO, can solve the problems of high cost, difficulty in ion exchange to achieve purity, low yield, etc., to reduce production cost and energy consumption, The effect of long effective drug concentration time and improving the purity standard
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Reaction and detection of EPO and GLUC-PEG
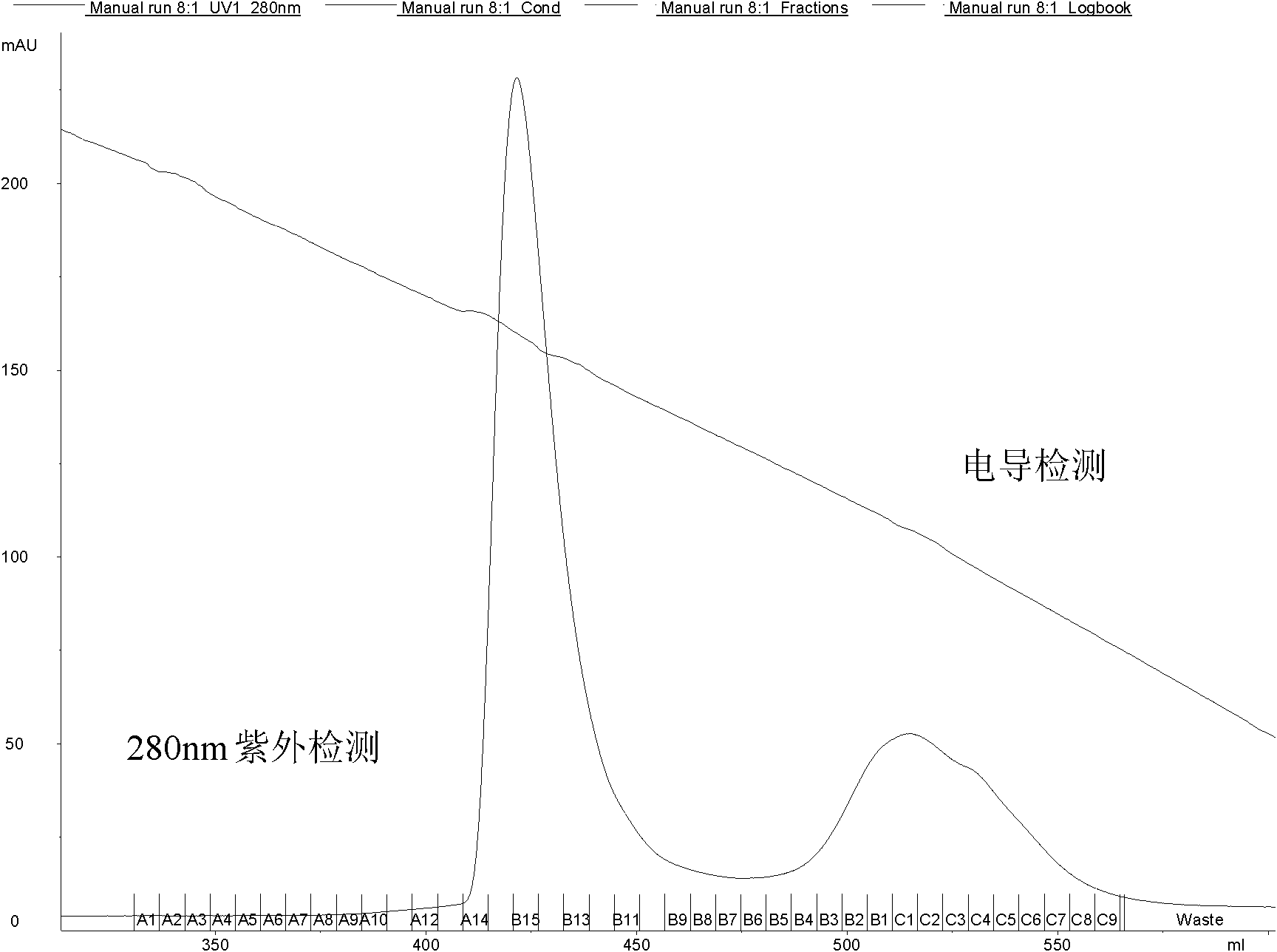
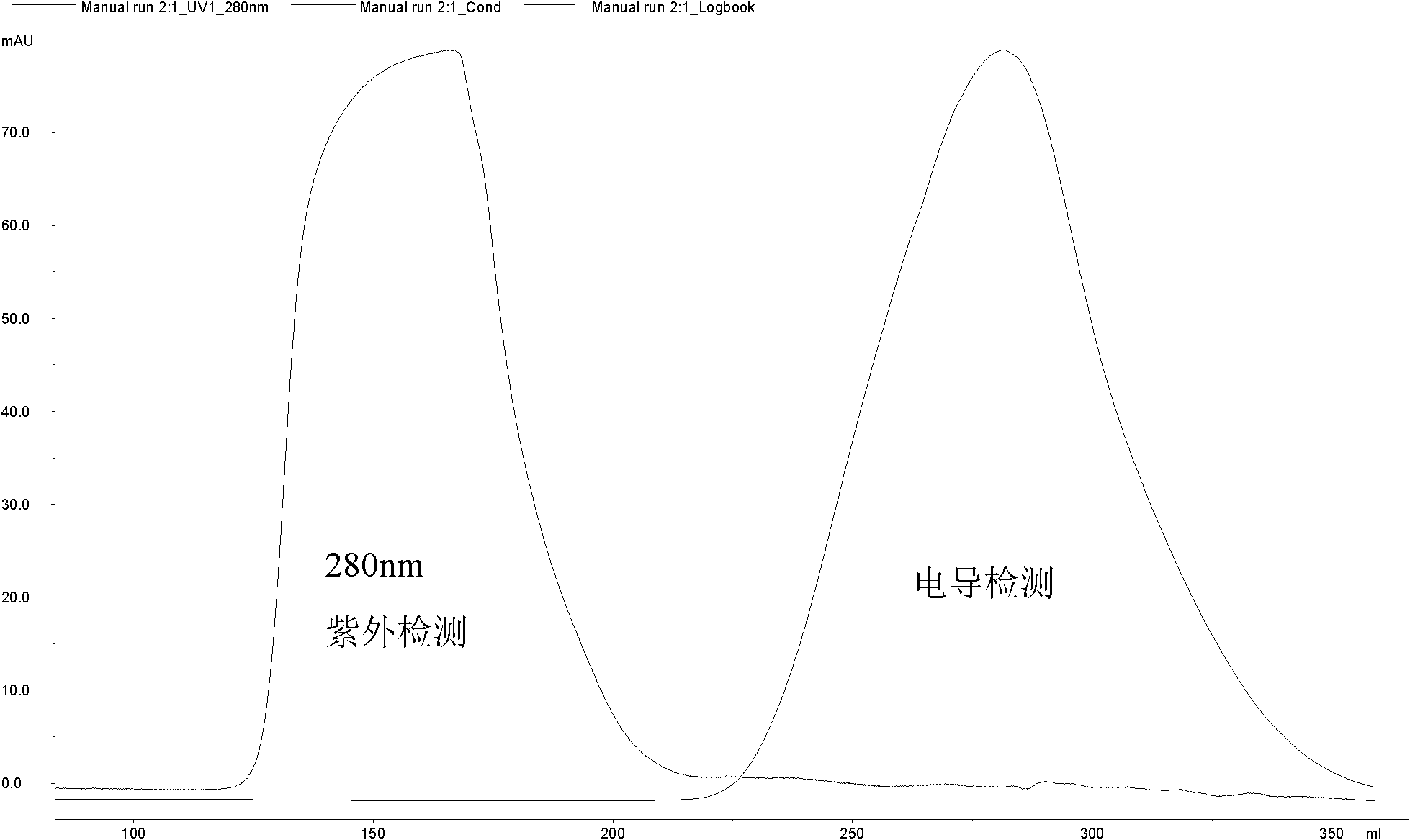
[0061] Use a 15ml plastic centrifuge tube, add 2ml of EPO stock solution (EPO content 2.5mg / ml, from Shenzhen Saibaoer Biological Pharmaceutical Co., Ltd.), add 3ml of 20mM phosphate buffer solution with pH 7.2, and the final concentration of EPO is 1mg / ml. Accurately weigh 109.4mg GLUC-PEG (35,000±3,500D, from Beijing Jiankai, see patent number: 02818455.6, structural formula as formula II) with an analytical balance, add 1094μl 2mM and pH 3.0 phosphate buffer to dissolve. After dissolving, all the GLUC-PEG-NHS solution was added into the EPO, and the pH of the EPO solution was measured to be 7.2 by test paper. The reaction mixture was placed on a shaker and reacted with shaking at room temperature for 2.5 hours.
[0062] After the reaction is completed, the reactant contains unreacted EPO, PEG, and the monosubstituted PEG-EPO (m PEG-EPO), disubstituted PEG-EPO (d PEG-EPO) and multi-substituted PEG-EPO (oliPEG-EPO) generate...
experiment example 1
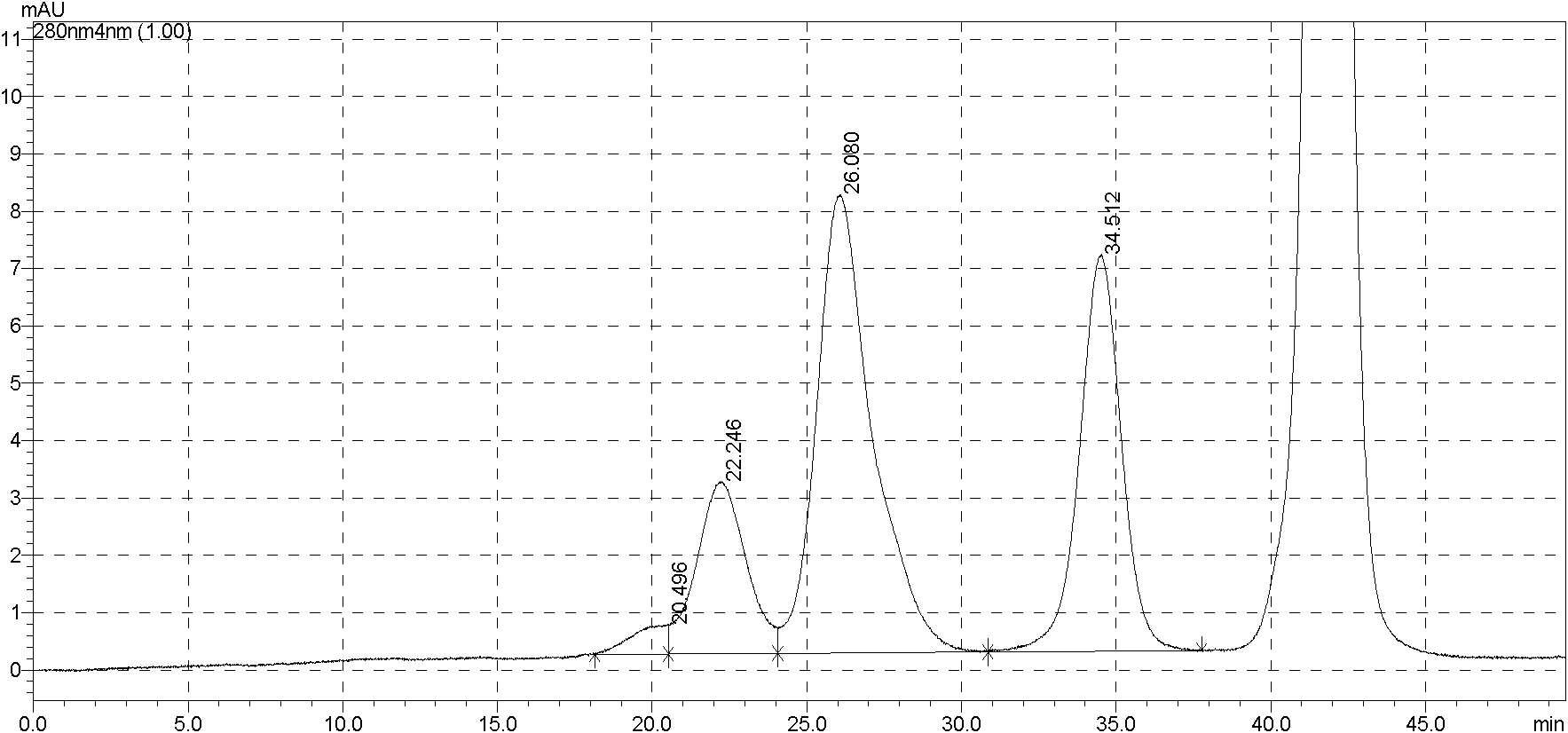
[0101] Purity detection of purified product mPEG-EPO (SDS-PAGE and SEC-HPLC)
[0102] SDS-PAGE (non-reduced) purity (according to the 2010 Pharmacopoeia method) detection of the purified product obtained in the above examples, 10 μg of conventional EPO and mPEG-EPO were loaded, and Coomassie brilliant blue staining, the results are as follows Figure 5 shown.
[0103] From Figure 5 It can be seen from the results that the mPEG-EPO purified by the above chromatography (hydrophobic + desalting + ion exchange + ultrafiltration) is the only band on the SDS-PAGE gel, and the purity is greater than 98%.
[0104] SEC-HPLC analysis is carried out to purified product, and condition is the same as embodiment 1, and result is as follows Figure 6 shown.
[0105]
[0106] It can be seen from the above results that the mPEG-EPO purified by the above chromatography (hydrophobic + desalting + ion exchange + ultrafiltration) was subjected to SEC-HPLC purity analysis, and the purity rea...
experiment example 2
[0109] In vivo activity detection of the purified product mPEG-EPO
[0110] Measuring principle: The proliferation and development process of the erythrocyte system in the bone marrow: pluripotent stem cells → unipotent stem cells → primitive erythrocytes → promyeloid erythrocytes → mesenchymal erythrocytes → late immature erythrocytes → reticulocytes → mature erythrocytes. From the proliferation of primitive erythrocytes to the stage of late erythrocytes, a total of 3-4 divisions are required, which takes about 72 hours. The number of erythrocytes changes from one to 8-16, the nucleus changes from large to small and condensed, and the hemoglobin in the cytoplasm gradually increases. After metablastic cells no longer divide, the nuclei are expelled during development and become reticulocytes. Reticulocytes contain a small amount of ribonucleic acid (RNA) and become reticular when stained with brilliant tar blue, hence the name reticulocytes. Reticulocytes mature further, RNA ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com